T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells
- PMID: 11752460
- PMCID: PMC64996
- DOI: 10.1073/pnas.261570598
T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells
Abstract
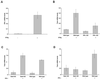
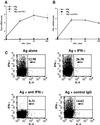
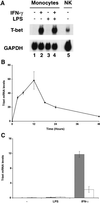
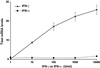
Differentiation of naive CD4(+) T cells into IFN-gamma-producing T helper 1 (T(H)1) cells is pivotal for protective immune responses against intracellular pathogens. T-bet, a recently discovered member of the T-box transcription factor family, has been reported to play a critical role in this process, promoting IFN-gamma production. Although terminal T(H)1 differentiation occurs over days, we now show that challenge of mice with a prototypical T(H)1-inducing stimulus, Toxoplasma gondii soluble extract, rapidly induced IFN-gamma and T-bet; T-bet induction was substantially lower in IFN-gamma-deficient mice. Naive T cells expressed little T-bet, but this transcription factor was induced markedly by the combination of IFN-gamma and cognate antigen. Human myeloid antigen-presenting cells showed T-bet induction after IFN-gamma stimulation alone, and this induction was antagonized by IL-4 and granulocyte/macrophage colony-stimulating factor. Although T-bet was induced rapidly and directly by IFN-gamma, it was not induced by IFN-alpha, lipopolysaccharide, or IL-1, indicating that this action of IFN-gamma was specific. Moreover, T-bet induction was dependent on Stat1 but not Stat4. These data argue for a model in which IFN-gamma gene regulation involves an autocrine loop, whereby the cytokine regulates a transcription factor that promotes its own production. These findings substantially alter the current view of T-bet in IFN-gamma regulation and promotion of cell-mediated immune responses.
Figures
References
-
- Abbas A K, Murphy K M, Sher A. Nature (London) 1996;383:787–793. - PubMed
-
- Murphy K M, Ouyang W, Farrar J D, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy T L. Annu Rev Immunol. 2000;18:451–494. - PubMed
-
- Jacobson N G, Szabo S J, Guler M L, Gorham J D, Murphy K M. Adv Exp Med Biol. 1996;409:61–73. - PubMed
-
- Kaplan M H, Sun Y L, Hoey T, Grusby M J. Nature (London) 1996;382:174–177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

