Vaccination with a Nogo-A-derived peptide after incomplete spinal-cord injury promotes recovery via a T-cell-mediated neuroprotective response: comparison with other myelin antigens
- PMID: 11752461
- PMCID: PMC65002
- DOI: 10.1073/pnas.011585298
Vaccination with a Nogo-A-derived peptide after incomplete spinal-cord injury promotes recovery via a T-cell-mediated neuroprotective response: comparison with other myelin antigens
Abstract
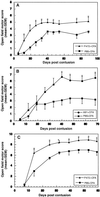
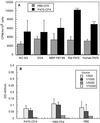
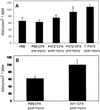
The myelin-associated protein Nogo-A has received more research attention than any other inhibitor of axonal regeneration in the injured central nervous system (CNS). Circumvention of its inhibitory effect, by using antibodies specific to Nogo-A, has been shown to promote axonal regrowth. Studies in our laboratory have demonstrated that active or passive immunization of CNS-injured rats or mice with myelin-associated peptides induces a T-cell-mediated protective autoimmune response, which promotes recovery by reducing posttraumatic degeneration. Here, we show that neuronal degeneration after incomplete spinal-cord contusion in rats was substantially reduced, and hence recovery was significantly promoted, by posttraumatic immunization with p472, a peptide derived from Nogo-A. The observed effect seemed to be mediated by T cells and could be reproduced by passive transfer of a T cell line directed against the Nogo-A peptide. Thus, it seems that after incomplete spinal-cord injury, immunization with a variety of myelin-associated peptides, including those derived from Nogo-A, can be used to evoke a T cell-mediated response that promotes recovery. The choice of peptide(s) for clinical treatment of spinal-cord injuries should be based on safety considerations; in particular, the likelihood that the chosen peptide will not cause an autoimmune disease or interfere with essential functions of this peptide or other proteins. From a therapeutic point of view, the fact that the active cellular agents are T cells rather than antibodies is an advantage, as T cell production commences within the time window required for a protective effect after spinal-cord injury, whereas antibody production takes longer.
Figures



Similar articles
-
Rapid induction of autoantibodies against Nogo-A and MOG in the absence of an encephalitogenic T cell response: implication for immunotherapeutic approaches in neurological diseases.FASEB J. 2003 Dec;17(15):2275-7. doi: 10.1096/fj.02-1203fje. Epub 2003 Oct 16. FASEB J. 2003. PMID: 14563689
-
Posttraumatic therapeutic vaccination with modified myelin self-antigen prevents complete paralysis while avoiding autoimmune disease.J Clin Invest. 2001 Aug;108(4):591-9. doi: 10.1172/JCI12837. J Clin Invest. 2001. PMID: 11518733 Free PMC article.
-
Nogo-66 receptor antagonist peptide promotes axonal regeneration.Nature. 2002 May 30;417(6888):547-51. doi: 10.1038/417547a. Nature. 2002. PMID: 12037567
-
The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review.
-
T cell-based therapeutic vaccination for spinal cord injury.Prog Brain Res. 2002;137:401-6. doi: 10.1016/s0079-6123(02)37031-6. Prog Brain Res. 2002. PMID: 12440382 Review.
Cited by
-
Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system.Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15620-5. doi: 10.1073/pnas.232565399. Epub 2002 Nov 12. Proc Natl Acad Sci U S A. 2002. PMID: 12429857 Free PMC article.
-
Taking a bite out of spinal cord injury: do dental stem cells have the teeth for it?Cell Mol Life Sci. 2016 Apr;73(7):1413-37. doi: 10.1007/s00018-015-2126-5. Epub 2016 Jan 14. Cell Mol Life Sci. 2016. PMID: 26768693 Free PMC article. Review.
-
Neural stem cell niches in health and diseases.Curr Pharm Des. 2012;18(13):1755-83. doi: 10.2174/138161212799859611. Curr Pharm Des. 2012. PMID: 22394166 Free PMC article. Review.
-
Quantitative 1H magnetic resonance spectroscopic imaging determines therapeutic immunization efficacy in an animal model of Parkinson's disease.J Neurosci. 2005 Feb 16;25(7):1691-700. doi: 10.1523/JNEUROSCI.4364-04.2005. J Neurosci. 2005. PMID: 15716405 Free PMC article.
-
Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats.J Neurosci. 2004 Apr 14;24(15):3752-61. doi: 10.1523/JNEUROSCI.0406-04.2004. J Neurosci. 2004. PMID: 15084655 Free PMC article.
References
-
- Schwab M E, Bartholdi D. Physiol Rev. 1996;76:319–370. - PubMed
-
- Bandtlow C E, Schwab M E. Glia. 2000;29:175–181. - PubMed
-
- Brittis P A, Flanagan J G. Neuron. 2001;30:11–14. - PubMed
-
- Fournier A E, Strittmatter S M. Curr Opin Neurobiol. 2001;11:89–94. - PubMed
-
- Huber A B, Schwab M E. Biol Chem Hoppe-Seyler. 2000;381:407–419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

