Delayed-onset ataxia in mice lacking alpha -tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress
- PMID: 11752462
- PMCID: PMC65004
- DOI: 10.1073/pnas.261456098
Delayed-onset ataxia in mice lacking alpha -tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress
Abstract
alpha-Tocopherol transfer protein (alpha-TTP) maintains the concentration of serum alpha-tocopherol (vitamin E), one of the most potent fat-soluble antioxidants, by facilitating alpha-tocopherol export from the liver. Mutations of the alpha-TTP gene are linked to ataxia with isolated vitamin E deficiency (AVED). We produced a model mouse of AVED by deleting the alpha-TTP gene, which showed ataxia and retinal degeneration after 1 year of age. Because the brain alpha-TTP functions in maintaining alpha-tocopherol levels in the brain, alpha-tocopherol was completely depleted in the alpha-TTP(-/-) mouse brain, and the neurological phenotype of alpha-TTP(-/-) mice is much more severe than that of wild-type mice when maintained on an alpha-tocopherol-deficient diet. Lipid peroxidation in alpha-TTP(-/-) mice brains showed a significant increase, especially in degenerating neurons. alpha-Tocopherol supplementation suppressed lipid peroxidation and almost completely prevented the development of neurological symptoms. This therapy almost completely corrects the abnormalities in a mouse model of human neurodegenerative disease. Moreover, alpha-TTP(-/-) mice may prove to be excellent animal models of delayed onset, slowly progressive neuronal degeneration caused by chronic oxidative stress.
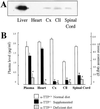
Figures





Similar articles
-
Alpha-tocopherol transfer protein deficiency in mice causes multi-organ deregulation of gene networks and behavioral deficits with age.Ann N Y Acad Sci. 2004 Dec;1031:109-26. doi: 10.1196/annals.1331.012. Ann N Y Acad Sci. 2004. PMID: 15753139
-
Molecular determinants of heritable vitamin E deficiency.Biochemistry. 2004 Apr 13;43(14):4143-9. doi: 10.1021/bi0363073. Biochemistry. 2004. PMID: 15065857
-
Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein.Nat Genet. 1995 Feb;9(2):141-5. doi: 10.1038/ng0295-141. Nat Genet. 1995. PMID: 7719340
-
The alpha-tocopherol transfer protein.Vitam Horm. 2007;76:45-65. doi: 10.1016/S0083-6729(07)76003-X. Vitam Horm. 2007. PMID: 17628171 Review.
-
Structure and function of alpha-tocopherol transfer protein: implications for vitamin E metabolism and AVED.Vitam Horm. 2007;76:23-43. doi: 10.1016/S0083-6729(07)76002-8. Vitam Horm. 2007. PMID: 17628170 Review.
Cited by
-
Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration.J Lipid Res. 2010 Mar;51(3):451-67. doi: 10.1194/jlr.R002238. Epub 2009 Sep 29. J Lipid Res. 2010. PMID: 19797256 Free PMC article. Review.
-
Evaluation of lovastatin effects on expression of anti-apoptotic Nrf2 and PGC-1α genes in neural stem cells treated with hydrogen peroxide.Mol Neurobiol. 2014 Jun;49(3):1364-72. doi: 10.1007/s12035-013-8613-5. Epub 2014 Jan 5. Mol Neurobiol. 2014. PMID: 24390568
-
RedEfish: Generation of the Polycistronic mScarlet: GSG-T2A: Ttpa Zebrafish Line.Antioxidants (Basel). 2021 Jun 16;10(6):965. doi: 10.3390/antiox10060965. Antioxidants (Basel). 2021. PMID: 34208660 Free PMC article.
-
Depletion of vitamin E increases amyloid beta accumulation by decreasing its clearances from brain and blood in a mouse model of Alzheimer disease.J Biol Chem. 2009 Nov 27;284(48):33400-8. doi: 10.1074/jbc.M109.054056. Epub 2009 Aug 13. J Biol Chem. 2009. PMID: 19679659 Free PMC article.
-
Regulation of the alpha-tocopherol transfer protein in mice: lack of response to dietary vitamin E or oxidative stress.Lipids. 2006 Feb;41(2):105-12. doi: 10.1007/s11745-006-5077-7. Lipids. 2006. PMID: 17707975
References
-
- Ben Hamida C, Doerflinger N, Belal S, Linder C, Reutenauer L, Dib C, Gyapay G, Vignal A, Le Paslier D, Cohen D. Nat Genet. 1993;5:195–200. - PubMed
-
- Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R, Arai H, Inoue K, Mandel J L, Koenig M. Nat Genet. 1995;9:141–145. - PubMed
-
- Gotoda T, Arita M, Arai H, Inoue K, Yokota T, Fukuo Y, Yazaki Y, Yamada N. N Engl J Med. 1995;333:1313–1318. - PubMed
-
- Yokota T, Shiojiri T, Gotoda T, Arai H. N Engl J Med. 1996;335:1770–1771. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

