Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease
- PMID: 11752464
- PMCID: PMC65009
- DOI: 10.1073/pnas.221292098
Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease
Abstract
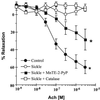
Plasma xanthine oxidase (XO) activity was defined as a source of enhanced vascular superoxide (O(2)( *-)) and hydrogen peroxide (H(2)O(2)) production in both sickle cell disease (SCD) patients and knockout-transgenic SCD mice. There was a significant increase in the plasma XO activity of SCD patients that was similarly reflected in the SCD mouse model. Western blot and enzymatic analysis of liver tissue from SCD mice revealed decreased XO content. Hematoxylin and eosin staining of liver tissue of knockout-transgenic SCD mice indicated extensive hepatocellular injury that was accompanied by increased plasma content of the liver enzyme alanine aminotransferase. Immunocytochemical and enzymatic analysis of XO in thoracic aorta and liver tissue of SCD mice showed increased vessel wall and decreased liver XO, with XO concentrated on and in vascular luminal cells. Steady-state rates of vascular O(2)( *-) production, as indicated by coelenterazine chemiluminescence, were significantly increased, and nitric oxide (( *)NO)-dependent vasorelaxation of aortic ring segments was severely impaired in SCD mice, implying oxidative inactivation of ( *)NO. Pretreatment of aortic vessels with the superoxide dismutase mimetic manganese 5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)porphyrin markedly decreased O(2)( small middle dot-) levels and significantly restored acetylcholine-dependent relaxation, whereas catalase had no effect. These data reveal that episodes of intrahepatic hypoxia-reoxygenation associated with SCD can induce the release of XO into the circulation from the liver. This circulating XO can then bind avidly to vessel luminal cells and impair vascular function by creating an oxidative milieu and catalytically consuming (*)NO via O(2)( small middle dot-)-dependent mechanisms.
Figures



Comment in
-
Reaping of nitric oxide by sickle cell disease.Proc Natl Acad Sci U S A. 2002 Jan 22;99(2):552-3. doi: 10.1073/pnas.032633399. Proc Natl Acad Sci U S A. 2002. PMID: 11805311 Free PMC article. No abstract available.
Similar articles
-
L-4F, an apolipoprotein A-1 mimetic, dramatically improves vasodilation in hypercholesterolemia and sickle cell disease.Circulation. 2003 May 13;107(18):2337-41. doi: 10.1161/01.CIR.0000070589.61860.A9. Epub 2003 May 5. Circulation. 2003. PMID: 12732610
-
Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits.Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8745-9. doi: 10.1073/pnas.93.16.8745. Proc Natl Acad Sci U S A. 1996. PMID: 8710942 Free PMC article.
-
Role of increased production of superoxide anions by NAD(P)H oxidase and xanthine oxidase in prolonged endotoxemia.Hypertension. 1999 May;33(5):1243-9. doi: 10.1161/01.hyp.33.5.1243. Hypertension. 1999. PMID: 10334819
-
Oxidant-mediated impairment of nitric oxide signaling in sickle cell disease--mechanisms and consequences.Cell Mol Biol (Noisy-le-grand). 2004 Feb;50(1):95-105. Cell Mol Biol (Noisy-le-grand). 2004. PMID: 15040433 Review.
-
Sickle cell disease: role of reactive oxygen and nitrogen metabolites.Clin Exp Pharmacol Physiol. 2007 Sep;34(9):926-32. doi: 10.1111/j.1440-1681.2007.04639.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17645642 Review.
Cited by
-
ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis.Front Immunol. 2022 Nov 1;13:1039241. doi: 10.3389/fimmu.2022.1039241. eCollection 2022. Front Immunol. 2022. PMID: 36389728 Free PMC article. Review.
-
The role of the arginine metabolome in pain: implications for sickle cell disease.J Pain Res. 2016 Mar 30;9:167-75. doi: 10.2147/JPR.S55571. eCollection 2016. J Pain Res. 2016. PMID: 27099528 Free PMC article. Review.
-
Decoding the role of SOD2 in sickle cell disease.Blood Adv. 2019 Sep 10;3(17):2679-2687. doi: 10.1182/bloodadvances.2019000527. Blood Adv. 2019. PMID: 31506286 Free PMC article. Review.
-
Peroxiredoxin-2 recycling is slower in denser and pediatric sickle cell red cells.FASEB J. 2022 Apr;36(4):e22267. doi: 10.1096/fj.202200052R. FASEB J. 2022. PMID: 35306694 Free PMC article.
-
Genetic deletion of apolipoprotein A-I increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung.J Lipid Res. 2010 Sep;51(9):2560-70. doi: 10.1194/jlr.M004549. Epub 2010 May 24. J Lipid Res. 2010. PMID: 20498409 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

