Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: the indirect antioxidant effects of sulforaphane
- PMID: 11752465
- PMCID: PMC65010
- DOI: 10.1073/pnas.261572998
Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: the indirect antioxidant effects of sulforaphane
Abstract
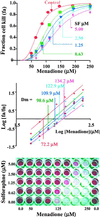
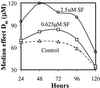
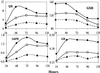
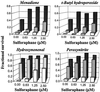
Mammalian cells are equipped with elaborate systems for protection against the toxicity of reactive oxygen and nitrogen species and electrophiles that are constant dangers to the integrity of their DNA. Phase 2 enzymes (e.g., glutathione transferases, NAD(P)H:quinone reductase) and glutathione synthesis are widely recognized as playing major protective roles against electrophilic carcinogens, but their antioxidant functions have attracted far less attention. The cytotoxicities of four oxidative stressors (menadione, tert-butyl hydroperoxide, 4-hydroxynonenal, and peroxynitrite) for human adult retinal pigment epithelial cells (ARPE-19) were quantified by measuring the concentration dependence of cell death and were expressed as the median effect dose (D(m)) for each oxidant. After treatment of ARPE-19 cells for 24 h with 0-5 microM concentrations of sulforaphane (the powerful Phase 2 enzyme inducer isolated from broccoli), the toxicities of the oxidants were markedly reduced as shown by 1.5- to 3-fold increases in D(m) values. The magnitude of protection was a function of the nature of the oxidants and the concentrations of both the oxidants and sulforaphane. Protection was prolonged and persisted for several days after removal of sulforaphane before returning to control levels. The sulforaphane-dependent increases in specific activities of cytosolic quinone reductase and the glutathione levels were highly significantly correlated with the degree of protection as measured by D(m) values. Antioxidant protection was also demonstrated for human HaCaT keratinocytes and L1210 murine leukemia cells. It is therefore highly likely that the multifaceted and prolonged antioxidant protection provided by sulforaphane is a general phenomenon that is mediated through induction of the Phase 2 enzyme response.
Figures

Similar articles
-
Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage.Proc Natl Acad Sci U S A. 2004 Jul 13;101(28):10446-51. doi: 10.1073/pnas.0403886101. Epub 2004 Jun 30. Proc Natl Acad Sci U S A. 2004. PMID: 15229324 Free PMC article.
-
Indirect antioxidant protection against photooxidative processes initiated in retinal pigment epithelial cells by a lipofuscin pigment.Rejuvenation Res. 2006 Summer;9(2):256-63. doi: 10.1089/rej.2006.9.256. Rejuvenation Res. 2006. PMID: 16706653
-
Aqueous extracts of selenium-fertilized broccoli increase selenoprotein activity and inhibit DNA single-strand breaks, but decrease the activity of quinone reductase in Hepa 1c1c7 cells.Food Chem Toxicol. 2006 May;44(5):695-703. doi: 10.1016/j.fct.2005.10.002. Epub 2005 Dec 22. Food Chem Toxicol. 2006. PMID: 16377050
-
Direct and indirect antioxidant properties of inducers of cytoprotective proteins.Mol Nutr Food Res. 2008 Jun;52 Suppl 1:S128-38. doi: 10.1002/mnfr.200700195. Mol Nutr Food Res. 2008. PMID: 18327872 Review.
-
Protective effect of sulforaphane against oxidative stress: recent advances.Exp Toxicol Pathol. 2012 Jul;64(5):503-8. doi: 10.1016/j.etp.2010.11.005. Epub 2010 Dec 3. Exp Toxicol Pathol. 2012. PMID: 21129940 Review.
Cited by
-
Sulforaphane induction of p21(Cip1) cyclin-dependent kinase inhibitor expression requires p53 and Sp1 transcription factors and is p53-dependent.J Biol Chem. 2012 May 11;287(20):16168-78. doi: 10.1074/jbc.M111.305292. Epub 2012 Mar 15. J Biol Chem. 2012. PMID: 22427654 Free PMC article.
-
Progesterone, Lipoic Acid, and Sulforaphane as Promising Antioxidants for Retinal Diseases: A Review.Antioxidants (Basel). 2019 Mar 2;8(3):53. doi: 10.3390/antiox8030053. Antioxidants (Basel). 2019. PMID: 30832304 Free PMC article. Review.
-
The Diversity of Chemoprotective Glucosinolates in Moringaceae (Moringa spp.).Sci Rep. 2018 May 22;8(1):7994. doi: 10.1038/s41598-018-26058-4. Sci Rep. 2018. PMID: 29789618 Free PMC article.
-
The Antimicrobial Effects of Myrosinase Hydrolysis Products Derived from Glucosinolates Isolated from Lepidium draba.Plants (Basel). 2024 Mar 30;13(7):995. doi: 10.3390/plants13070995. Plants (Basel). 2024. PMID: 38611524 Free PMC article.
-
The emerging role of Nrf2 in mitochondrial function.Free Radic Biol Med. 2015 Nov;88(Pt B):179-188. doi: 10.1016/j.freeradbiomed.2015.04.036. Epub 2015 May 11. Free Radic Biol Med. 2015. PMID: 25975984 Free PMC article. Review.
References
-
- Halliwell B, Gutteridge J M C. Free Radicals in Biology and Medicine. New York: Oxford Univ. Press; 1999. pp. 1–36.
-
- Pokorny J, Yanishlieva M, Gordon M. Antioxidants in Food: Practical Applications. Cambridge, U.K.: Woodhead Publishing, Ltd.; 2001.
-
- Talalay P, Fahey J W, Holtzclaw W D, Prestera T, Zhang Y. Toxicol Lett. 1995;82/83:173–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

