Sulfhydryl modification of V449C in the glutamate transporter EAAT1 abolishes substrate transport but not the substrate-gated anion conductance
- PMID: 11752470
- PMCID: PMC65028
- DOI: 10.1073/pnas.011400198
Sulfhydryl modification of V449C in the glutamate transporter EAAT1 abolishes substrate transport but not the substrate-gated anion conductance
Abstract
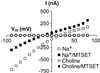
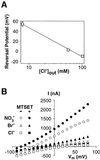
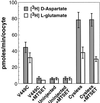
Excitatory amino acid transporters (EAATs) buffer and remove synaptically released L-glutamate and maintain its concentrations below neurotoxic levels. EAATs also mediate a thermodynamically uncoupled substrate-gated anion conductance that may modulate cell excitability. Here, we demonstrate that modification of a cysteine substituted within a C-terminal domain of EAAT1 abolishes transport in both the forward and reverse directions without affecting activation of the anion conductance. EC(50)s for L-glutamate and sodium are significantly lower after modification, consistent with kinetic models of the transport cycle that link anion channel gating to an early step in substrate translocation. Also, decreasing the pH from 7.5 to 6.5 decreases the EC(50) for L-glutamate to activate the anion conductance, without affecting the EC(50) for the entire transport cycle. These findings demonstrate for the first time a structural separation of transport and the uncoupled anion flux. Moreover, they shed light on some controversial aspects of the EAAT transport cycle, including the kinetics of proton binding and anion conductance activation.
Figures

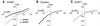

Similar articles
-
A Mutation in Transmembrane Domain 7 (TM7) of Excitatory Amino Acid Transporters Disrupts the Substrate-dependent Gating of the Intrinsic Anion Conductance and Drives the Channel into a Constitutively Open State.J Biol Chem. 2015 Sep 18;290(38):22977-90. doi: 10.1074/jbc.M115.660860. Epub 2015 Jul 22. J Biol Chem. 2015. PMID: 26203187 Free PMC article.
-
Mutations in transmembrane domains 5 and 7 of the human excitatory amino acid transporter 1 affect the substrate-activated anion channel.Biochemistry. 2007 Aug 28;46(34):9685-92. doi: 10.1021/bi700647f. Epub 2007 Aug 4. Biochemistry. 2007. PMID: 17676873
-
Distinct conformational states mediate the transport and anion channel properties of the glutamate transporter EAAT-1.J Biol Chem. 2002 Apr 19;277(16):13494-500. doi: 10.1074/jbc.M109970200. Epub 2002 Jan 28. J Biol Chem. 2002. PMID: 11815608
-
Molecular Basis of Coupled Transport and Anion Conduction in Excitatory Amino Acid Transporters.Neurochem Res. 2022 Jan;47(1):9-22. doi: 10.1007/s11064-021-03252-x. Epub 2021 Feb 15. Neurochem Res. 2022. PMID: 33587237 Free PMC article. Review.
-
Functional diversity of excitatory amino acid transporters: ion channel and transport modes.Am J Physiol. 1999 Oct;277(4):F481-6. doi: 10.1152/ajprenal.1999.277.4.F481. Am J Physiol. 1999. PMID: 10516269 Review.
Cited by
-
Aspartate-444 is essential for productive substrate interactions in a neuronal glutamate transporter.J Gen Physiol. 2007 Jun;129(6):527-39. doi: 10.1085/jgp.200609707. J Gen Physiol. 2007. PMID: 17535962 Free PMC article.
-
Structure and function of sodium-coupled GABA and glutamate transporters.J Membr Biol. 2006;213(2):89-100. doi: 10.1007/s00232-006-0877-5. Epub 2007 Apr 6. J Membr Biol. 2006. PMID: 17417704 Review.
-
Substrate transport and anion permeation proceed through distinct pathways in glutamate transporters.Elife. 2017 Jun 1;6:e25850. doi: 10.7554/eLife.25850. Elife. 2017. PMID: 28569666 Free PMC article.
-
The conserved histidine 295 does not contribute to proton cotransport by the glutamate transporter EAAC1.Biochemistry. 2005 Mar 8;44(9):3466-76. doi: 10.1021/bi047812i. Biochemistry. 2005. PMID: 15736956 Free PMC article.
-
Modulation of glutamate and glycine transporters by niflumic, flufenamic and mefenamic acids.Neurochem Res. 2009 Oct;34(10):1738-47. doi: 10.1007/s11064-009-9983-y. Epub 2009 May 15. Neurochem Res. 2009. PMID: 19444608
References
-
- Dingledine R, Borges K, Bowie D, Traynelis S F. Pharmacol Rev. 1999;51:7–61. - PubMed
-
- Choi D W. Prog Brain Res. 1994;100:47–51. - PubMed
-
- Nicholls D, Attwell D. Trends Pharmacol Sci. 1990;11:462–468. - PubMed
-
- Seal R P, Amara S G. Annu Rev Pharmacol Toxicol. 1999;39:431–456. - PubMed
-
- Kanner B I, Sharon I. Biochemistry. 1978;17:3949–3953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

