The 2.6 A resolution structure of Rhodobacter capsulatus bacterioferritin with metal-free dinuclear site and heme iron in a crystallographic 'special position'
- PMID: 11752777
- PMCID: PMC4615704
- DOI: 10.1107/s0907444901017267
The 2.6 A resolution structure of Rhodobacter capsulatus bacterioferritin with metal-free dinuclear site and heme iron in a crystallographic 'special position'
Abstract
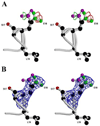
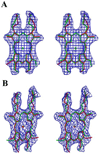
Bacterioferritin from Rhodobacter capsulatus was crystallized and its structure was solved at 2.6 A resolution. This first structure of a bacterioferritin from a photosynthetic organism is a spherical particle of 24 subunits displaying 432 point-group symmetry like ferritin and bacterioferritin from Escherichia coli. Crystallized in the I422 space group, its structural analysis reveals for the first time the non-symmetric heme molecule located on a twofold crystallographic symmetry axis. Other hemes of the protomer are situated on twofold noncrystallographic axes. Apparently, both types of sites bind heme in two orientations, leading to an average structure consisting of a symmetric 50:50 mixture, thus satisfying the crystallographic and noncrystallographic symmetry of the crystal. Five water molecules are situated close to the heme, which is bound in a hydrophobic pocket and axially coordinated by two crystallographic or noncrystallographically related methionine residues. Its ferroxidase center, in which Fe(II) is oxidized to Fe(III), is empty or fractionally occupied by a metal ion. Two positions are observed for the coordinating Glu18 side chain instead of one in the E. coli enzyme in which the site is occupied. This result suggests that the orientation of the Glu18 side chain could be constrained by this interaction.
Figures



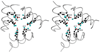
Similar articles
-
Serendipitous crystallization and structure determination of bacterioferritin from Achromobacter.Acta Crystallogr F Struct Biol Commun. 2018 Sep 1;74(Pt 9):558-566. doi: 10.1107/S2053230X18009809. Epub 2018 Aug 29. Acta Crystallogr F Struct Biol Commun. 2018. PMID: 30198888 Free PMC article.
-
The binding of haem and zinc in the 1.9 A X-ray structure of Escherichia coli bacterioferritin.J Biol Inorg Chem. 2009 Feb;14(2):201-7. doi: 10.1007/s00775-008-0438-8. Epub 2008 Oct 23. J Biol Inorg Chem. 2009. PMID: 18946693
-
Iron metabolism in Rhodobacter capsulatus. Characterisation of bacterioferritin and formation of non-haem iron particles in intact cells.Eur J Biochem. 1994 Aug 1;223(3):847-55. doi: 10.1111/j.1432-1033.1994.tb19061.x. Eur J Biochem. 1994. PMID: 8055962
-
Iron storage in bacteria.Adv Microb Physiol. 1998;40:281-351. doi: 10.1016/s0065-2911(08)60134-4. Adv Microb Physiol. 1998. PMID: 9889981 Review.
-
Bacterioferritin: a hemoprotein member of the ferritin family.Adv Exp Med Biol. 1994;356:157-64. doi: 10.1007/978-1-4615-2554-7_18. Adv Exp Med Biol. 1994. PMID: 7887220 Review. No abstract available.
Cited by
-
Bis-methionyl coordination in the crystal structure of the heme-binding domain of the streptococcal cell surface protein Shp.J Mol Biol. 2007 Nov 23;374(2):374-83. doi: 10.1016/j.jmb.2007.08.058. Epub 2007 Aug 31. J Mol Biol. 2007. PMID: 17920629 Free PMC article.
-
Unveiling Structural Heterogeneity and Evolutionary Adaptations of Heteromultimeric Bacterioferritin Nanocages.Adv Sci (Weinh). 2025 May;12(20):e2409957. doi: 10.1002/advs.202409957. Epub 2025 Apr 1. Adv Sci (Weinh). 2025. PMID: 40167232 Free PMC article.
-
Bacterioferritin from Mycobacterium smegmatis contains zinc in its di-nuclear site.Protein Sci. 2008 Jul;17(7):1138-50. doi: 10.1110/ps.034819.108. Epub 2008 Apr 29. Protein Sci. 2008. PMID: 18445621 Free PMC article.
-
Protein dynamics and ion traffic in bacterioferritin.Biochemistry. 2012 Dec 11;51(49):9900-10. doi: 10.1021/bi3013388. Epub 2012 Nov 30. Biochemistry. 2012. PMID: 23167635 Free PMC article.
-
Iron homeostasis in the Rhodobacter genus.Adv Bot Res. 2013;66:10.1016/B978-0-12-397923-0.00010-2. doi: 10.1016/B978-0-12-397923-0.00010-2. Adv Bot Res. 2013. PMID: 24382933 Free PMC article.
References
-
- Harrison PM, Arosio P. Biochim. Biophys. Acta. 1996;1275:161–203. - PubMed
-
- Andrews SC, Le Brun N, Barynin V, Thomson AJ, Moore GR, Guest JR, Harrison PM. J. Biol. Chem. 1995;270:23268–23274. - PubMed
-
- Romao CV, Louro R, Timkovich R, Lubben M, Liu MY, LeGall J, Xavier AV, Teixeira M. FEBS Lett. 2000;480:213–216. - PubMed
-
- Frolow F, Gilboa AJK, Yariv J. Nat. Struc. Biol. 1994;1:453–460. - PubMed
-
- Yang X, Le Brun NE, Thomson AJ, Moore GR, Chasteen ND. Biochemistry. 2000;39:4915–4923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

