Biophysics of radiofrequency ablation using an irrigated electrode
- PMID: 11752906
- PMCID: PMC5779086
- DOI: 10.1023/a:1013224110550
Biophysics of radiofrequency ablation using an irrigated electrode
Abstract
Background: Previous reports have proposed that prevention of electrode-endocardial interfacial boiling is the key mechanism by which radiofrequency application using an irrigated electrode yields a larger ablation lesion than a non-irrigated electrode. It has been suggested that maximal myocardial temperature is shifted deep into myocardium during irrigated ablation.
Purpose: To examine the biophysics of irrigated ablation by correlating electrode and myocardial temperatures with ablation circuit impedance and lesion morphology, and to perform a comparison with non-irrigated ablation modes. To assess the influence of irrigant rate, composition, temperature and blood flow velocity.
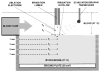
Methods: I. Ablation with and without electrode irrigation was performed in vitro utilizing a whole blood-superfused system. Electrode, electrode-endocardial interface, and intramyocardial temperatures were assessed, as were ablation circuit impedance, total delivered energy, and lesion and electrode morphology. Irrigants assessed were room temperature normal saline, iced normal saline, and dextrose. Irrigant flow rates assessed were 20 and 100 cc/min. Blood flow velocities assessed were 0 and 0.26 m/s. II. Finite element simulations of myocardial temperature during irrigated ablation were performed to further elucidate irrigation biophysics and provide a more detailed myocardial temperature profile. Two models were constructed, each utilizing a different core assumption regarding the electrode-tissue boundary: 1. electrode temperature measured in vitro; 2. interfacial temperature measured in vitro. Intramyocardial temperatures predicted by each model were correlated with corresponding temperatures measured in vitro.
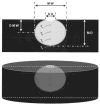
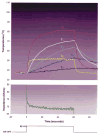
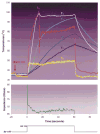
Results: I. Ablation during electrode irrigation with normal saline was associated with greater ablation energy deposition and larger lesion dimensions than non-irrigated ablation. The mechanism underlying the larger lesion was delay or inhibition of impedance rise; this was associated with attenuation or prevention of electrode coagulum. Irrigation did not prevent interfacial boiling, which occurred during uninterrupted radiofrequency energy deposition and lesion growth. Irrigation using saline at 100 cc/min was associated with no impedance rise regardless of blood flow velocity, whereas during irrigation at 20 cc/min impedance rise was blood flow rate-dependent. Iced saline produced results equivalent to room temperature saline. Irrigation with dextrose was associated with curtailed energy application and relatively small lesions. II. The finite element simulation that used electrode-endocardial interfacial temperature as the core assumption predicted a myocardial temperature profile which correlated significantly better with in vitro than did the simulation which used electrode temperature as the core assumption. Regardless of irrigant and blood flow rates, maximal myocardial temperature was always within 1 mm of the endocardial surface.
Conclusions: Radiofrequency energy application via a saline irrigated electrode resulted in a larger lesion due to attenuation or eradication of electrode coagulum, thus preventing an impedance rise. Irrigation did not prevent interfacial boiling, but boiling did not prevent lesion growth. The site of maximal myocardial temperature during irrigated ablation was relatively superficial, always within 1 mm of the endocardial surface. Irrigation with iced saline was no more effective than with room temperature saline; both were far more effective than dextrose. Higher irrigation rates immunized the electrode from the influence of blood flow. The biophysical effects of blood flow and irrigation were similar.
Figures




References
-
- Mittleman RS, Huang SKS, DeGuzman WT, Cuenoud H, Wagshal AB, Pires LA. Use of the saline infusion electrode catheter for improved energy delivery and increased lesion size in radiofrequency catheter ablation. Pacing Clin Electrophysiol. 1995;18:1022–1027. - PubMed
-
- Skrumeda LL, Mehra R. Comparison of standard and irrigated radiofrequency ablation in the canine ventricle. J Cardiovasc Electrophysiol. 1998;9:1196–1205. - PubMed
-
- Ruffy R, Imran MA, Santel DJ, Wharton JM. Radio-frequency delivery through a cooled catheter tip allows the creation of larger endomyocardial lesions in the ovine heart. J Cardiovasc Electrophysiol. 1995;6:1089–1096. - PubMed
-
- Peterson HH, Chen X, Pietersen A, Svendsen JH, Haunso S. Tissue temperatures and lesion size during irrigated tip catheter radiofrequency ablation. Pacing Clin Electrophysiol. 2000;23:8–17. - PubMed
-
- Nakagawa H, Yamanashi WS, Pitha JV, Arruda M, Wang X, Ohtomo K, Beckman KJ, McClelland JH, Lazzara R, Jackman WM. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation. 1995;91:2264–2273. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
