Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis
- PMID: 11753040
- PMCID: PMC1422393
- DOI: 10.1097/00000658-200201000-00005
Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis
Abstract
Objective: To investigate the existence of a pathway between intraluminal products and the muscularis leukocytic infiltrate.
Summary background data: Mild intestinal manipulation or lipopolysaccharide initiates an intense inflammatory response within the intestinal muscularis, resulting in paralytic ileus. A major potential morbidity factor in ileus is luminal bacterial overgrowth.
Methods: ACI rats were subjected to small bowel manipulation, after which fluorescent carboxylated or paramagnetic microspheres were administered into the gut lumen. Animals were killed between 0 and 24 hours; unoperated rats served as controls.
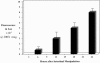
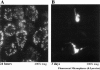
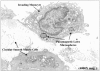
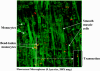
Results: Intestinal manipulation led to an early transient transference of microspheres from the intestinal lumen into mesenteric lymph that was not observed in unmanipulated controls. A time- dependent, significant increase in microsphere-laden phagocytes was observed within the intestinal muscularis. Immunohistochemistry and electron microscopy of the intestinal muscularis identified the phagocytes as extravasating ED1+ monocytes. Interruption of the lymphatics abolished the accumulation of microsphere-laden monocytes within the muscularis, although a significant monocytic recruitment could still be observed within the intestinal muscularis.
Conclusions: These data show that intestinal manipulation leads to a transient increase in mucosal permeability and that the extraintestinal endocytotic uptake of transferred particles by circulating monocytes precedes their infiltration into the gut wall. The transference of luminal bacterial products may follow a similar route and time course as the microspheres. The authors hypothesize that endogenous bacterial products act synergistically with the inflammatory response within the postsurgical intestinal muscularis, leading to an exacerbation of postoperative ileus.
Figures






References
-
- Bates DW, Sands K, Miller E, et al. Predicting bacteremia in patients with sepsis syndrome. J Infect Dis 1997; 176: 1538–1551. - PubMed
-
- Livingston DH, Mosenthal AC, Deitch EA. Sepsis and multiple organ dysfunction syndrome: a clinical-mechanistic overview. New Horizons 1995; 3: 257–266. - PubMed
-
- Pape H-C, Dwenger A, Regel G, et al. Increased gut permeability after multiple trauma. Br J Surg 1994; 81: 850–852. - PubMed
-
- Saadia R. Trauma and bacterial translocation. Br J Surg 1995; 82: 1243–1244. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

