Abelson kinase regulates epithelial morphogenesis in Drosophila
- PMID: 11756472
- PMCID: PMC2199330
- DOI: 10.1083/jcb.200105102
Abelson kinase regulates epithelial morphogenesis in Drosophila
Abstract
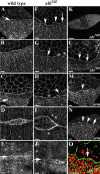
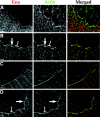
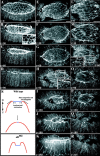
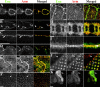
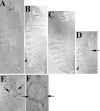
Activation of the nonreceptor tyrosine kinase Abelson (Abl) contributes to the development of leukemia, but the complex roles of Abl in normal development are not fully understood. Drosophila Abl links neural axon guidance receptors to the cytoskeleton. Here we report a novel role for Drosophila Abl in epithelial cells, where it is critical for morphogenesis. Embryos completely lacking both maternal and zygotic Abl die with defects in several morphogenetic processes requiring cell shape changes and cell migration. We describe the cellular defects that underlie these problems, focusing on dorsal closure as an example. Further, we show that the Abl target Enabled (Ena), a modulator of actin dynamics, is involved with Abl in morphogenesis. We find that Ena localizes to adherens junctions of most epithelial cells, and that it genetically interacts with the adherens junction protein Armadillo (Arm) during morphogenesis. The defects of abl mutants are strongly enhanced by heterozygosity for shotgun, which encodes DE-cadherin. Finally, loss of Abl reduces Arm and alpha-catenin accumulation in adherens junctions, while having little or no effect on other components of the cytoskeleton or cell polarity machinery. We discuss possible models for Abl function during epithelial morphogenesis in light of these data.
Figures




References
-
- Bashaw, G.J., T. Kidd, D. Murray, T. Pawson, and C.S. Goodman. 2000. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 101:703–715. - PubMed
-
- Baum, B., and N. Perrimon. 2001. Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat. Cell Biol. 3:883–890. - PubMed
-
- Bear, J.E., J.J. Loureiro, I. Libova, R. Fassler, J. Wehland, and F.B. Gertler. 2000. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 101:717–728. - PubMed
-
- Bennett, R.L., and F.M. Hoffmann. 1992. Increased levels of the Drosophila Abelson tyrosine kinase in nerves and muscles: Subcellular localization and mutant phenotypes imply a role in cell-cell interactions. Development. 116:953–966. - PubMed
-
- Brown, N.H., S.L. Gregory, and M.D. Martin-Bermudo. 2000. Integrins as mediators of morphogenesis in Drosophila. Dev. Biol. 223:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

