Small cargo proteins and large aggregates can traverse the Golgi by a common mechanism without leaving the lumen of cisternae
- PMID: 11756473
- PMCID: PMC2199327
- DOI: 10.1083/jcb.200108073
Small cargo proteins and large aggregates can traverse the Golgi by a common mechanism without leaving the lumen of cisternae
Abstract
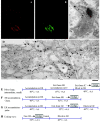
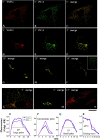
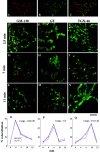
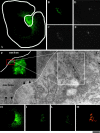
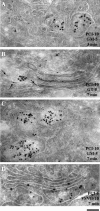
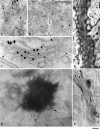
Procollagen (PC)-I aggregates transit through the Golgi complex without leaving the lumen of Golgi cisternae. Based on this evidence, we have proposed that PC-I is transported across the Golgi stacks by the cisternal maturation process. However, most secretory cargoes are small, freely diffusing proteins, thus raising the issue whether they move by a transport mechanism different than that used by PC-I. To address this question we have developed procedures to compare the transport of a small protein, the G protein of the vesicular stomatitis virus (VSVG), with that of the much larger PC-I aggregates in the same cell. Transport was followed using a combination of video and EM, providing high resolution in time and space. Our results reveal that PC-I aggregates and VSVG move synchronously through the Golgi at indistinguishable rapid rates. Additionally, not only PC-I aggregates (as confirmed by ultrarapid cryofixation), but also VSVG, can traverse the stack without leaving the cisternal lumen and without entering Golgi vesicles in functionally relevant amounts. Our findings indicate that a common mechanism independent of anterograde dissociative carriers is responsible for the traffic of small and large secretory cargo across the Golgi stack.
Figures


Similar articles
-
Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation.Cell. 1998 Dec 23;95(7):993-1003. doi: 10.1016/s0092-8674(00)81723-7. Cell. 1998. PMID: 9875853
-
Traffic through the Golgi apparatus.J Cell Biol. 2001 Dec 24;155(7):1099-101. doi: 10.1083/jcb.200110160. Epub 2001 Dec 24. J Cell Biol. 2001. PMID: 11756463 Free PMC article. Review.
-
Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport.J Cell Biol. 2001 Dec 24;155(7):1213-24. doi: 10.1083/jcb.200108029. Epub 2001 Dec 17. J Cell Biol. 2001. PMID: 11748250 Free PMC article.
-
Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells.J Cell Biol. 1998 Dec 14;143(6):1485-503. doi: 10.1083/jcb.143.6.1485. J Cell Biol. 1998. PMID: 9852146 Free PMC article.
-
A cisternal maturation mechanism can explain the asymmetry of the Golgi stack.FEBS Lett. 1997 Sep 8;414(2):177-81. doi: 10.1016/s0014-5793(97)00984-8. FEBS Lett. 1997. PMID: 9315681 Review.
Cited by
-
Golgi function and dysfunction in the first COG4-deficient CDG type II patient.Hum Mol Genet. 2009 Sep 1;18(17):3244-56. doi: 10.1093/hmg/ddp262. Epub 2009 Jun 3. Hum Mol Genet. 2009. PMID: 19494034 Free PMC article.
-
The KDEL receptor signalling cascade targets focal adhesion kinase on focal adhesions and invadopodia.Oncotarget. 2017 Dec 19;9(12):10228-10246. doi: 10.18632/oncotarget.23421. eCollection 2018 Feb 13. Oncotarget. 2017. PMID: 29535802 Free PMC article.
-
Group IV phospholipase A(2)alpha controls the formation of inter-cisternal continuities involved in intra-Golgi transport.PLoS Biol. 2009 Sep;7(9):e1000194. doi: 10.1371/journal.pbio.1000194. Epub 2009 Sep 15. PLoS Biol. 2009. PMID: 19753100 Free PMC article.
-
Conformational defects slow Golgi exit, block oligomerization, and reduce raft affinity of caveolin-1 mutant proteins.Mol Biol Cell. 2004 Oct;15(10):4556-67. doi: 10.1091/mbc.e04-06-0480. Epub 2004 Aug 10. Mol Biol Cell. 2004. PMID: 15304521 Free PMC article.
-
Characterization of a mammalian Golgi-localized protein complex, COG, that is required for normal Golgi morphology and function.J Cell Biol. 2002 Apr 29;157(3):405-15. doi: 10.1083/jcb.200202016. Epub 2002 Apr 29. J Cell Biol. 2002. PMID: 11980916 Free PMC article.
References
-
- Becker, B., B. Bolinger, and M. Melkonian. 1995. Anterograde transport of algal scales through the Golgi complex is not mediated by vesicles. Trends Cell Biol. 5:305–307. - PubMed
-
- Bergmann, J.E. 1989. Using temperature-sensitive mutants of VSV to study membrane protein biogenesis. Methods Cell Biol. 32:85–110. - PubMed
-
- Bonfanti, L., A.A. Mironov, Jr., J.A. Martinez-Menarguez, O. Martella, A. Fusella, M. Baldassarre, R. Buccione, H.J. Geuze, A.A. Mironov, and A. Luini. 1998. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 95:993–1003. - PubMed
-
- Bruckner, P., and E.E. Eikenberry. 1984. Procollagen is more stable in cellulo than in vitro. Eur. J. Biochem. 140:397–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

