Human VPS34 is required for internal vesicle formation within multivesicular endosomes
- PMID: 11756475
- PMCID: PMC2199316
- DOI: 10.1083/jcb.200108152
Human VPS34 is required for internal vesicle formation within multivesicular endosomes
Abstract
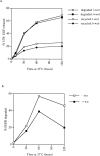
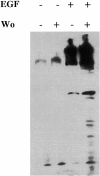
After internalization from the plasma membrane, activated EGF receptors (EGFRs) are delivered to multivesicular bodies (MVBs). Within MVBs, EGFRs are removed from the perimeter membrane to internal vesicles, thereby being sorted from transferrin receptors, which recycle back to the plasma membrane. The phosphatidylinositol (PI) 3'-kinase inhibitor, wortmannin, inhibits internal vesicle formation within MVBs and causes EGFRs to remain in clusters on the perimeter membrane. Microinjection of isotype-specific inhibitory antibodies demonstrates that the PI 3'-kinase required for internal vesicle formation is hVPS34. In the presence of wortmannin, EGFRs continue to be delivered to lysosomes, showing that their removal from the recycling pathway and their delivery to lysosomes does not depend on inward vesiculation. We showed previously that tyrosine kinase-negative EGFRs fail to accumulate on internal vesicles of MVBs but are recycled rather than delivered to lysosomes. Therefore, we conclude that selection of EGFRs for inclusion on internal vesicles requires tyrosine kinase but not PI 3'-kinase activity, whereas vesicle formation requires PI 3'-kinase activity. Finally, in wortmannin-treated cells there is increased EGF-stimulated tyrosine phosphorylation when EGFRs are retained on the perimeter membrane of MVBs. Therefore, we suggest that inward vesiculation is involved directly with attenuating signal transduction.
Figures






References
-
- Bright, N.A., B.J. Reaves, B.M. Mullock, and J.P. Luzio. 1997. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J. Cell Sci. 110:2027–2040. - PubMed
-
- Bright, N.A., M.R. Lindsay, A. Stewart, and J.P. Luzio. 2001. The relationship between lumenal and limiting membranes in swollen late endocytic compartments formed after wortmannin treatment or sucrose accumulation. Traffic. 2:631–642. - PubMed
-
- Chin, L.-S., M.C. Raynor, X. Wei, H.-Q. Chen, and L. Li. 2001. Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J. Biol. Chem. 276:7069–7078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

