Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP
- PMID: 11756479
- PMCID: PMC2199346
- DOI: 10.1083/jcb.200102017
Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP
Abstract
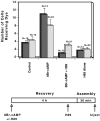
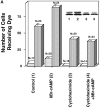
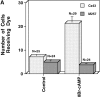
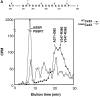
The assembly of gap junctions (GJs) is a process coordinated by growth factors, kinases, and other signaling molecules. GJ assembly can be enhanced via the elevation of cAMP and subsequent stimulation of connexon trafficking to the plasma membrane. To study the positive regulation of GJ assembly, fibroblasts derived from connexin (Cx)43 knockout (KO) and wild-type (WT) mice were transfected with WT Cx43 (WTCx43) or mutant Cx43. GJ assembly between untransfected WT fibroblasts or stably transfected WTCx43/KO fibroblasts was increased two- to fivefold by 8Br-cAMP, and this increase could be blocked by inhibition of cAMP-dependent protein kinase (PKA) or truncation of the Cx43 COOH terminus (CT). Although serine 364 (S364) of the Cx43 CT was determined to be a major site of phosphorylation, the molar ratio of Cx43 phosphorylation was not increased by 8Br-cAMP. Importantly, GJ assembly between either S364ECx43/KO or S364ECx43/WT fibroblasts was stimulated by 8Br-cAMP, but that between S364ACx43/KO or S364PCx43/KO fibroblasts was not stimulated, indicating that phosphorylation or a negative charge at S364 is required for enhancement of GJ assembly by cAMP. Furthermore, GJ assembly between S364ACx43/WT fibroblasts could be stimulated by 8Br-cAMP, but could not be between S364PCx43/WT fibroblasts. Thus, S364PCx43 interferes with enhanced GJ assembly when coexpressed with WTCx43.
Figures










Similar articles
-
Gap junction regulation of vascular tone: implications of modulatory intercellular communication during gestation.Adv Exp Med Biol. 2014;814:117-32. doi: 10.1007/978-1-4939-1031-1_11. Adv Exp Med Biol. 2014. PMID: 25015806 Free PMC article. Review.
-
Cyclic AMP and LDL trigger a rapid enhancement in gap junction assembly through a stimulation of connexin trafficking.J Cell Sci. 2000 Sep;113 ( Pt 17):3037-49. doi: 10.1242/jcs.113.17.3037. J Cell Sci. 2000. PMID: 10934042
-
Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes.Circ Res. 2005 Sep 30;97(7):655-62. doi: 10.1161/01.RES.0000183880.49270.f9. Epub 2005 Aug 25. Circ Res. 2005. PMID: 16123333
-
Cyclic Nucleotides Differentially Regulate Cx43 Gap Junction Function in Uterine Artery Endothelial Cells From Pregnant Ewes.Hypertension. 2017 Aug;70(2):401-411. doi: 10.1161/HYPERTENSIONAHA.117.09113. Epub 2017 May 30. Hypertension. 2017. PMID: 28559397 Free PMC article.
-
Misregulation of connexin43 gap junction channels and congenital heart defects.Novartis Found Symp. 1999;219:212-21; discussion 221-5. doi: 10.1002/9780470515587.ch13. Novartis Found Symp. 1999. PMID: 10207906 Review.
Cited by
-
Adrenocortical Gap Junctions and Their Functions.Front Endocrinol (Lausanne). 2016 Jun 29;7:82. doi: 10.3389/fendo.2016.00082. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27445985 Free PMC article. Review.
-
Effects of cyclic AMP on the function of the cardiac gap junction during hypoxia.Exp Clin Cardiol. 2006 Winter;11(4):286-93. Exp Clin Cardiol. 2006. PMID: 18651019 Free PMC article.
-
Gap junction regulation of vascular tone: implications of modulatory intercellular communication during gestation.Adv Exp Med Biol. 2014;814:117-32. doi: 10.1007/978-1-4939-1031-1_11. Adv Exp Med Biol. 2014. PMID: 25015806 Free PMC article. Review.
-
Cholesterol metabolism and Cx43, Cx46, and Cx50 gap junction protein expression and localization in normal and diabetic and obese ob/ob and db/db mouse testes.Am J Physiol Endocrinol Metab. 2018 Jan 1;314(1):E21-E38. doi: 10.1152/ajpendo.00215.2017. Epub 2017 Aug 29. Am J Physiol Endocrinol Metab. 2018. PMID: 28851737 Free PMC article.
-
Gap junctions.Compr Physiol. 2012 Jul;2(3):1981-2035. doi: 10.1002/cphy.c110051. Compr Physiol. 2012. PMID: 23723031 Free PMC article. Review.
References
-
- Atkinson, M.M., P.D. Lampe, H.H. Lin, R. Kollander, X.R. Li, and D.T. Kiang. 1995. Cyclic AMP modifies the cellular distribution of connexin43 and induces a persistent increase in the junctional permeability of mouse mammary tumor cells. J. Cell Sci. 108:3079–3090. - PubMed
-
- Beardslee, M.A., J.G. Laing, E.C. Beyer, and J.E. Saffitz. 1998. Rapid turnover of connexin43 in the adult rat heart. Circ. Res. 83:629–635. - PubMed
-
- Berthoud, V.M., M.B. Rook, O. Traub, E.L. Hertzberg, and J.C. Saez. 1993. On the mechanisms of cell uncoupling induced by a tumor promoter phorbol ester in clone 9 cells, a rat liver epithelial cell line. Eur. J. Cell Biol. 62:384–396. - PubMed
-
- Berthoud, V.M., E.C. Beyer, W.E. Kurata, A.F. Lau, and P.D. Lampe. 1997. The gap-junction protein connexin 56 is phosphorylated in the intracellular loop and the carboxy-terminal region. Eur. J. Biochem. 244:89–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

