Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior
- PMID: 11756480
- PMCID: PMC2199321
- DOI: 10.1083/jcb.200107107
Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior
Abstract
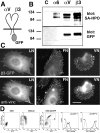
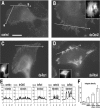
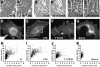
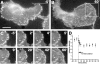
Integrins are cell-substrate adhesion molecules that provide the essential link between the actin cytoskeleton and the extracellular matrix during cell migration. We have analyzed alphaVbeta3-integrin dynamics in migrating cells using a green fluorescent protein-tagged beta3-integrin chain. At the cell front, adhesion sites containing alphaVbeta3-integrin remain stationary, whereas at the rear of the cell they slide inward. The integrin fluorescence intensity within these different focal adhesions, and hence the relative integrin density, is directly related to their mobility. Integrin density is as much as threefold higher in sliding compared with stationary focal adhesions. High intracellular tension under the control of RhoA induced the formation of high-density contacts. Low-density adhesion sites were induced by Rac1 and low intracellular tension. Photobleaching experiments demonstrated a slow turnover of beta3-integrins in low-density contacts, which may account for their stationary nature. In contrast, the fast beta3-integrin turnover observed in high-density contacts suggests that their apparent sliding may be caused by a polarized renewal of focal contacts. Therefore, differential acto-myosin-dependent integrin turnover and focal adhesion densities may explain the mechanical and behavioral differences between cell adhesion sites formed at the front, and those that move in the retracting rear of migrating cells.
Figures




References
-
- Albelda, S.M., S.A. Mette, D.E. Elder, R. Stewart, L. Damjanovich, M. Herlyn, and C.A. Buck. 1990. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 50:6757–6764. - PubMed
-
- Amano, M., K. Chihara, K. Kimura, Y. Fukata, N. Nakamura, Y. Matsuura, and K. Kaibuchi. 1997. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 275:1308–1311. - PubMed
-
- Anderson, K.I., and R. Cross. 2000. Contact dynamics during keratocyte motility. Curr. Biol. 10:253–260. - PubMed
-
- Balaban, N.Q., U.S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

