Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-beta peptide exposure: involvement of Src family protein kinases
- PMID: 11756483
- PMCID: PMC6757621
- DOI: 10.1523/JNEUROSCI.22-01-00010.2002
Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-beta peptide exposure: involvement of Src family protein kinases
Abstract
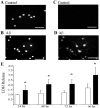
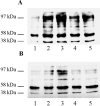
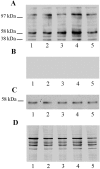
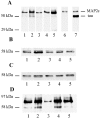
The increased production of amyloid beta-peptide (Abeta) in Alzheimer's disease is acknowledged to be a key pathogenic event. In this study, we examined the response of primary human and rat brain cortical cultures to Abeta administration and found a marked increase in the tyrosine phosphorylation content of numerous neuronal proteins, including tau and putative microtubule-associated protein 2c (MAP2c). We also found that paired helical filaments of aggregated and hyperphosphorylated tau are tyrosine phosphorylated, indicating that changes in the phosphotyrosine content of cytoplasmic proteins in response to Abeta are potentially an important process. Increased tyrosine phosphorylation of cytoskeletal and other neuronal proteins was specific to fibrillar Abeta(25-35) and Abeta(1-42). The tyrosine phosphorylation was blocked by addition of the Src family tyrosine kinase inhibitor 4-amino-5-(4-chlorophenyl)-7(t-butyl)pyrazol(3,4-d)pyramide (PP2) and the phosphatidylinositol 3-kinase inhibitor LY 294002. Tyrosine phosphorylation of tau and MAP2c was concomitant with an increase in the tyrosine phosphorylation and subsequent putative activation of the non-receptor kinase, focal adhesion kinase (FAK). Immunoprecipitation of Fyn, a member of the Src family, from Abeta(25-35)-treated neurons showed an increased association of Fyn with FAK. Abeta treatment of cells also stimulated the sustained activation of extracellular regulated kinase-2, which was blocked by addition of PP2 and LY 294002, suggesting that FAK/Fyn/PI3-kinase association is upstream of mitogen-activated protein (MAP) kinase signaling in Abeta-treated neurons. This cascade of signaling events contains the earliest biochemical changes in neurons to be described in response to Abeta exposure and may be critical for subsequent neurodegenerative changes.
Figures

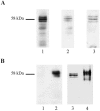

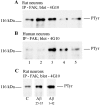

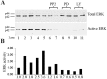
References
-
- Abe K, Saito H. Amyloid beta neurotoxicity not mediated by the mitogen-activated protein kinase cascade in cultured rat hippocampal and cortical neurons. Neurosci Lett. 2000;292:1–4. - PubMed
-
- Alvarez A, Toro R, Caceres A, Maccioni RB. Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Lett. 1999;459:421–426. - PubMed
-
- Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS Lett. 1999;453:260–264. - PubMed
-
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
