Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses
- PMID: 11756484
- PMCID: PMC6757598
- DOI: 10.1523/JNEUROSCI.22-01-00021.2002
Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses
Abstract
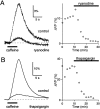
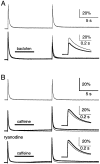
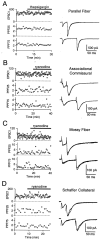
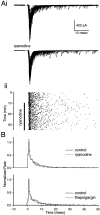
Recent evidence suggests that internal calcium stores and calcium-induced calcium release (CICR) provide an important source of calcium that drives short-term presynaptic plasticity at central synapses. Here we tested for the involvement of CICR in short-term presynaptic plasticity at six excitatory synapses in acute rat hippocampal and cerebellar brain slices. Depletion of internal calcium stores with thapsigargin and prevention of CICR with ryanodine have no effect on paired-pulse facilitation, delayed release of neurotransmitter, or calcium-dependent recovery from depression. Fluorometric calcium measurements also show that these drugs have no effect on the residual calcium signal that underlies these forms of short-term presynaptic plasticity. Finally, although caffeine causes CICR in Purkinje cell bodies and dendrites, it does not elicit CICR in parallel fiber inputs to these cells. Taken together, these results indicate that for the excitatory synapses studied here, internal calcium stores and CICR do not contribute to short-term presynaptic plasticity on the milliseconds-to-seconds time scale. Instead, this plasticity is driven by the residual calcium signal arising from calcium entry through voltage-gated calcium channels.
Figures

Similar articles
-
Activation of presynaptic and postsynaptic ryanodine-sensitive calcium stores is required for the induction of long-term depression at GABAergic synapses in the neonatal rat hippocampus.J Neurosci. 2000 Sep 1;20(17):RC94. doi: 10.1523/JNEUROSCI.20-17-j0002.2000. J Neurosci. 2000. PMID: 10952733 Free PMC article.
-
Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release.Neuron. 2001 Jan;29(1):197-208. doi: 10.1016/s0896-6273(01)00190-8. Neuron. 2001. PMID: 11182091
-
Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse.J Neurosci. 2003 Dec 3;23(35):11229-34. doi: 10.1523/JNEUROSCI.23-35-11229.2003. J Neurosci. 2003. PMID: 14657182 Free PMC article.
-
Stores not just for storage. intracellular calcium release and synaptic plasticity.Neuron. 2001 Aug 30;31(4):519-22. doi: 10.1016/s0896-6273(01)00402-0. Neuron. 2001. PMID: 11545711 Review.
-
Short-term forms of presynaptic plasticity.Curr Opin Neurobiol. 2011 Apr;21(2):269-74. doi: 10.1016/j.conb.2011.02.003. Epub 2011 Feb 23. Curr Opin Neurobiol. 2011. PMID: 21353526 Free PMC article. Review.
Cited by
-
Assessing the role of GLUK5 and GLUK6 at hippocampal mossy fiber synapses.J Neurosci. 2004 Nov 10;24(45):10093-8. doi: 10.1523/JNEUROSCI.3078-04.2004. J Neurosci. 2004. PMID: 15537878 Free PMC article.
-
Functional integration of calcium regulatory mechanisms at Purkinje neuron synapses.Cerebellum. 2012 Sep;11(3):640-50. doi: 10.1007/s12311-010-0185-6. Cerebellum. 2012. PMID: 20596808 Free PMC article. Review.
-
Use-dependent amplification of presynaptic Ca2+ signaling by axonal ryanodine receptors at the hippocampal mossy fiber synapse.Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11998-2003. doi: 10.1073/pnas.0802175105. Epub 2008 Aug 7. Proc Natl Acad Sci U S A. 2008. PMID: 18687898 Free PMC article.
-
CaMKII inactivation by extracellular Ca(2+) depletion in dorsal root ganglion neurons.Cell Calcium. 2006 May;39(5):445-54. doi: 10.1016/j.ceca.2006.01.005. Epub 2006 Mar 7. Cell Calcium. 2006. PMID: 16519936 Free PMC article.
-
Contributions of SERCA pump and ryanodine-sensitive stores to presynaptic residual Ca2+.Cell Calcium. 2010 Apr;47(4):326-38. doi: 10.1016/j.ceca.2010.01.004. Epub 2010 Feb 13. Cell Calcium. 2010. PMID: 20153896 Free PMC article.
References
-
- Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
