Activity- and target-dependent regulation of large-conductance Ca2+-activated K+ channels in developing chick lumbar motoneurons
- PMID: 11756490
- PMCID: PMC6757614
- DOI: 10.1523/JNEUROSCI.22-01-00073.2002
Activity- and target-dependent regulation of large-conductance Ca2+-activated K+ channels in developing chick lumbar motoneurons
Abstract
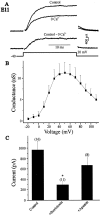
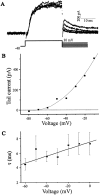
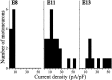
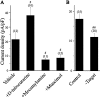
The functional expression of large-conductance (BK-type) Ca2+-activated K+ (K(Ca)) channels was examined in developing chick lumbar motoneurons (LMNs) between embryonic day 6 (E6) and E13 using patch-clamp recording techniques. The macroscopic K(Ca) current of E13 LMNs is inhibited by iberiotoxin and resistant to apamin. The average macroscopic K(Ca) density was low before E8 and increased 3.3-fold by E11, with an additional 1.8-fold increase occurring by E13. BK-type K(Ca) channels could not be detected in inside-out patches from E8 LMNs but were readily detected at E11. The density of voltage-activated Ca2+ currents did not change between E8 and E11. Surgical ablation of target tissues at E5 caused a significant reduction in average K(Ca) density in LMNs measured at E11. Conversely, chronic in ovo administration of d-tubocurarine, which causes an increase in motoneuron branching on the surface of the muscle target tissue, evoked a 1.8-fold increase in average LMN K(Ca) density measured at E11. Electrical activity also contributed to developmental regulation of LMN K(Ca) density. A significant reduction in E11 K(Ca) density was found after chronic in ovo treatment with the neuronal nicotinic antagonist mecamylamine or the GABA receptor agonist muscimol, agents that reduce activation of LMNs in ovo. Moreover, 3 d exposure to depolarizing concentrations of external K+ to LMNs cultured at E8 caused an increase in K(Ca) expression. Conversely, tetrodotoxin caused a decrease in K(Ca) expression in cultured E8 LMNs developing for 3 d in the presence of neurotrophic factors that promote neuronal survival in the absence of target tissues.
Figures




Similar articles
-
Glial cell line-derived neurotrophic factor and target-dependent regulation of large-conductance KCa channels in developing chick lumbar motoneurons.J Neurosci. 2002 Dec 1;22(23):10201-8. doi: 10.1523/JNEUROSCI.22-23-10201.2002. J Neurosci. 2002. PMID: 12451121 Free PMC article.
-
A-current expression is regulated by activity but not by target tissues in developing lumbar motoneurons of the chick embryo.J Neurophysiol. 2004 Nov;92(5):2644-51. doi: 10.1152/jn.00307.2004. Epub 2004 May 26. J Neurophysiol. 2004. PMID: 15163671
-
BK-Type K(Ca) channels in two parasympathetic cell types: differences in kinetic properties and developmental expression.J Neurophysiol. 2000 Dec;84(6):2767-76. doi: 10.1152/jn.2000.84.6.2767. J Neurophysiol. 2000. PMID: 11110807
-
Role of cell-cell interactions in the developmental regulation of Ca2+-activated K+ currents in vertebrate neurons.J Neurobiol. 1998 Oct;37(1):23-36. doi: 10.1002/(sici)1097-4695(199810)37:1<23::aid-neu3>3.0.co;2-a. J Neurobiol. 1998. PMID: 9777730 Review.
-
Role of organellar Ca2+-activated K+ channels in disease development.Life Sci. 2023 Mar 1;316:121433. doi: 10.1016/j.lfs.2023.121433. Epub 2023 Jan 25. Life Sci. 2023. PMID: 36708987 Review.
Cited by
-
Homeostatic synaptic plasticity in developing spinal networks driven by excitatory GABAergic currents.Neuropharmacology. 2014 Mar;78:55-62. doi: 10.1016/j.neuropharm.2013.04.058. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727439 Free PMC article. Review.
-
Glial cell line-derived neurotrophic factor and target-dependent regulation of large-conductance KCa channels in developing chick lumbar motoneurons.J Neurosci. 2002 Dec 1;22(23):10201-8. doi: 10.1523/JNEUROSCI.22-23-10201.2002. J Neurosci. 2002. PMID: 12451121 Free PMC article.
-
Elevated intracellular Na+ concentrations in developing spinal neurons.J Neurochem. 2017 Mar;140(5):755-765. doi: 10.1111/jnc.13936. Epub 2017 Jan 23. J Neurochem. 2017. PMID: 28027400 Free PMC article.
-
Inhibition of electrical activity by retroviral infection with Kir2.1 transgenes disrupts electrical differentiation of motoneurons.PLoS One. 2008 Aug 13;3(8):e2971. doi: 10.1371/journal.pone.0002971. PLoS One. 2008. PMID: 18698433 Free PMC article.
-
Pharmacological manipulation of GABA-driven activity in ovo disrupts the development of dendritic morphology but not the maturation of spinal cord network activity.Neural Dev. 2010 Apr 8;5:11. doi: 10.1186/1749-8104-5-11. Neural Dev. 2010. PMID: 20377848 Free PMC article.
References
-
- Becker E, Soler RM, Yuste VJ, Gine E, Sanz-Rodriguez C, Egea J, Martin-Zanca D, Comella JX. Development of survival responsiveness to brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5, but not to nerve growth factor, in cultured motoneurons from chick embryo spinal cord. J Neurosci. 1998;18:7903–7911. - PMC - PubMed
-
- Buonanno A, Fields RD. Gene regulation by patterned electrical activity during neural and skeletal muscle development. Curr Opin Neurobiol. 1999;9:110–120. - PubMed
-
- Cameron J, Dryer SE. BK-Type KCa channels in two parasympathetic cell types: differences in kinetic properties, developmental expression. J Neurophysiol. 2000;84:2767–2776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
