Inhibition of the c-Jun N-terminal kinase signaling pathway by the mixed lineage kinase inhibitor CEP-1347 (KT7515) preserves metabolism and growth of trophic factor-deprived neurons
- PMID: 11756493
- PMCID: PMC6757592
- DOI: 10.1523/JNEUROSCI.22-01-00103.2002
Inhibition of the c-Jun N-terminal kinase signaling pathway by the mixed lineage kinase inhibitor CEP-1347 (KT7515) preserves metabolism and growth of trophic factor-deprived neurons
Abstract
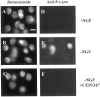
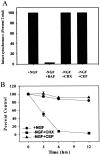
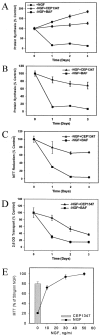
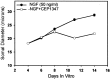
Nerve growth factor (NGF) deprivation triggers metabolic changes in sympathetic neurons that precede cell death. Here, we investigate the role of the c-Jun N-terminal kinase (JNK) pathway in downregulating neuronal metabolism. We show that, in the presence of CEP-1347 (KT7515), a small molecule known to block cell death upstream of JNK, cellular metabolism is preserved in neurons deprived of NGF. Biochemical data that are presented are consistent with the mechanism of action of CEP-1347 being the inhibition of the mixed lineage kinases (MLKs), known activators of JNK signaling. We demonstrate that CEP-1347-saved neurons continue to grow even in the absence of NGF, indicating that inhibition of the JNK pathway is permissive for neuronal growth in the absence of trophic support. These trophic effects are seen despite the fact that CEP-1347 does not stimulate several known survival kinase pathways. In addition to blocking Bax-dependent cytochrome c release, the inhibition of the JNK signaling pathway with CEP-1347 also blocks the development of competence-to-die in response to cytosolic cytochrome c. Therefore, inhibition of the JNK signaling pathway with the MLK inhibitor CEP-1347 inhibits both limbs of the apoptotic pathway. Finally, we demonstrate that neurons that have been NGF-deprived long-term but that have been kept alive by caspase inhibitors can be rescued metabolically by CEP-1347 as assessed by soma size, cytochrome c localization, and protein synthesis rates. Therefore, we conclude that, in addition to converting extracellular signals into decisions of life and death, the JNK pathway can modulate cellular metabolism directly and thereby maintain not only survival but the "quality of life" of neurons.
Figures






Similar articles
-
Identification of JNK-dependent and -independent components of cerebellar granule neuron apoptosis.J Neurochem. 2002 Nov;83(4):992-1001. doi: 10.1046/j.1471-4159.2002.01219.x. J Neurochem. 2002. PMID: 12421372
-
The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis.Mol Cell Biol. 2001 Jul;21(14):4713-24. doi: 10.1128/MCB.21.14.4713-4724.2001. Mol Cell Biol. 2001. PMID: 11416147 Free PMC article.
-
Mixed-lineage kinase inhibitors require the activation of Trk receptors to maintain long-term neuronal trophism and survival.J Pharmacol Exp Ther. 2005 Mar;312(3):1007-19. doi: 10.1124/jpet.104.077800. Epub 2004 Nov 3. J Pharmacol Exp Ther. 2005. PMID: 15525794
-
Mixed-lineage kinases: a target for the prevention of neurodegeneration.Annu Rev Pharmacol Toxicol. 2004;44:451-74. doi: 10.1146/annurev.pharmtox.44.101802.121840. Annu Rev Pharmacol Toxicol. 2004. PMID: 14744254 Review.
-
Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential.Biochim Biophys Acta. 2004 Mar 11;1697(1-2):89-101. doi: 10.1016/j.bbapap.2003.11.016. Biochim Biophys Acta. 2004. PMID: 15023353 Review.
Cited by
-
Proneurotrophin-3 is a neuronal apoptotic ligand: evidence for retrograde-directed cell killing.J Neurosci. 2009 Nov 25;29(47):14790-802. doi: 10.1523/JNEUROSCI.2059-09.2009. J Neurosci. 2009. PMID: 19940174 Free PMC article.
-
Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3.Mol Cell Biol. 2003 Sep;23(17):6027-36. doi: 10.1128/MCB.23.17.6027-6036.2003. Mol Cell Biol. 2003. PMID: 12917327 Free PMC article.
-
Programmed cell death during neuronal development: the sympathetic neuron model.Cell Death Differ. 2014 Jul;21(7):1025-35. doi: 10.1038/cdd.2014.47. Epub 2014 Apr 25. Cell Death Differ. 2014. PMID: 24769728 Free PMC article. Review.
-
Pin1 promotes cell death in NGF-dependent neurons through a mechanism requiring c-Jun activity.J Neurochem. 2008 Jul;106(2):734-45. doi: 10.1111/j.1471-4159.2008.05427.x. Epub 2008 Apr 14. J Neurochem. 2008. PMID: 18419764 Free PMC article.
-
ZPK/DLK, a mitogen-activated protein kinase kinase kinase, is a critical mediator of programmed cell death of motoneurons.J Neurosci. 2011 May 18;31(20):7223-8. doi: 10.1523/JNEUROSCI.5947-10.2011. J Neurosci. 2011. PMID: 21593306 Free PMC article.
References
-
- Borasio GD, Horstmann S, Anneser JM, Neff NT, Glicksman MA. CEP-1347/KT7515, a JNK pathway inhibitor, supports the in vitro survival of chick embryonic neurons. NeuroReport. 1998;9:1435–1439. - PubMed
-
- Deckwerth TL, Easton RM, Knudson CM, Korsmeyer SJ, Johnson EM., Jr Placement of the BCL-2 family member BAX in the death pathway of sympathetic neurons activated by trophic factor deprivation. Exp Neurol. 1998;152:150–162. - PubMed
-
- Deshmukh M, Johnson EM., Jr Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron. 1998;21:695–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
