Anti-S-nitrosocysteine antibodies are a predictive marker for demyelination in experimental autoimmune encephalomyelitis: implications for multiple sclerosis
- PMID: 11756495
- PMCID: PMC6757586
- DOI: 10.1523/JNEUROSCI.22-01-00123.2002
Anti-S-nitrosocysteine antibodies are a predictive marker for demyelination in experimental autoimmune encephalomyelitis: implications for multiple sclerosis
Abstract
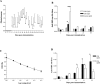
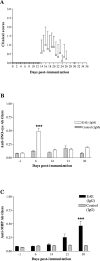
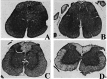
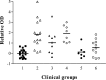
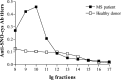
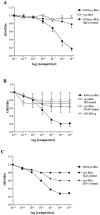
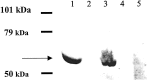
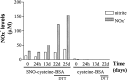
Multiple sclerosis (MS) is characterized by inflammation within the CNS. This inflammatory response is associated with production of nitric oxide (NO) and NO-related species that nitrosylate thiols. We postulated that MS patients would exhibit an antibody (Ab) response directed against proteins containing S-nitrosocysteine (SNO-cysteine) and showed that anti-NO-cysteine Abs of the IgM isotype are in fact present in the sera of some MS patients (Boullerne et al., 1995). We report here the presence of a seemingly identical Ab response directed against SNO-cysteine in an acute model of MS, experimental autoimmune encephalomyelitis (EAE) induced in Lewis rats with the 68-84 peptide of guinea pig myelin basic protein (MBP(68-84)). Serum levels of anti-SNO-cysteine Abs peaked 1 week before the onset of clinical signs and well before the appearance of anti-MBP(68-84) Abs. The anti-SNO-cysteine Ab peak titer correlated with the extent of subsequent CNS demyelination, suggesting a link between Ab level and CNS lesion formation. In relapsing-remitting MS patients, we found elevated anti-SNO-cysteine Ab at times of relapse and normal values in most patients judged to be in remission. Two-thirds of patients with secondary progressive MS had elevated anti-SNO-cysteine Ab levels, including those receiving interferon beta-1b. The data show that a rise in circulating anti-SNO-cysteine Ab levels precedes onset of EAE. Anti-SNO-cysteine Abs are also elevated at times of MS attacks and in progressive disease, suggesting a possible role for these Abs, measurable in blood, as a biological marker for clinical activity.
Figures

References
-
- Avrameas S, Ternynck T. The natural autoantibodies system: between hypotheses and facts. Mol Immunol. 1993;30:1133–1142. - PubMed
-
- Boullerne AI, Petry KG, Meynard M, Geffard M. Indirect evidence for nitric oxide involvement in multiple sclerosis by characterization of circulating antibodies directed against conjugated S-nitrosocysteine. J Neuroimmunol. 1995;60:117–124. - PubMed
-
- Boullerne AI, Nedelkoska L, Benjamins JA. Role of calcium in nitric oxide-induced cytotoxicity: EGTA protects mouse oligodendrocytes. J Neurosci Res. 2001;63:124–135. - PubMed
-
- Brundin L, Morcos E, Olsson T, Wiklund NP, Andersson M. Increased intrathecal nitric oxide formation in multiple sclerosis; cerebrospinal fluid nitrite as activity marker. Eur J Neurol. 1999;5:585–590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
