Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation
- PMID: 11756504
- PMCID: PMC6757620
- DOI: 10.1523/JNEUROSCI.22-01-00209.2002
Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation
Abstract
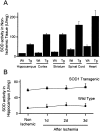
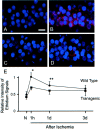
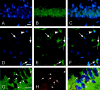
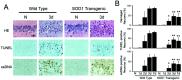
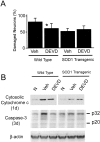
Mitochondria are known to be involved in the early stage of apoptosis by releasing cytochrome c, caspase-9, and the second mitochondria-derived activator of caspases (Smac). We have reported that overexpression of copper/zinc superoxide dismutase (SOD1) reduced superoxide production and ameliorated neuronal injury in the hippocampal CA1 subregion after global ischemia. However, the role of oxygen free radicals produced after ischemia/reperfusion in the mitochondrial signaling pathway has not been clarified. Five minutes of global ischemia was induced in male SOD1-transgenic (Tg) and wild-type (Wt) littermate rats. Cytosolic expression of cytochrome c and Smac and activation of caspases were evaluated by immunohistochemistry, Western blot, and caspase activity assay. Apoptotic cell death was characterized by DNA nick end and single-stranded DNA labeling. In the Wt animals, early superoxide production, mitochondrial release of cytochrome c, Smac, and cleaved caspase-9 were observed after ischemia. Active caspase-3 was subsequently increased, and 85% of the hippocampal CA1 neurons showed apoptotic DNA damage 3 d after ischemia. Tg animals showed less superoxide production and cytochrome c and Smac release. Subsequent active caspase-3 expression was not evident, and only 45% of the neurons showed apoptotic DNA damage. A caspase-3 inhibitor (N-benzyloxycarbonyl-val-ala-asp-fluoromethyl ketone) reduced cell death only in Wt animals. These results suggest that overexpression of SOD1 reduced oxidative stress, thereby attenuating the mitochondrial release of cytochrome c and Smac, resulting in less caspase activation and apoptotic cell death. Oxygen free radicals may play a pivotal role in the mitochondrial signaling pathway of apoptotic cell death in hippocampal CA1 neurons after global ischemia.
Figures


Similar articles
-
Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion.J Neurosci. 1998 Oct 15;18(20):8292-9. doi: 10.1523/JNEUROSCI.18-20-08292.1998. J Neurosci. 1998. PMID: 9763473 Free PMC article.
-
Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats.J Cereb Blood Flow Metab. 2006 Dec;26(12):1479-89. doi: 10.1038/sj.jcbfm.9600303. Epub 2006 Mar 15. J Cereb Blood Flow Metab. 2006. PMID: 16538228
-
Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release.FASEB J. 2002 Dec;16(14):1997-9. doi: 10.1096/fj.02-0251fje. Epub 2002 Oct 4. FASEB J. 2002. PMID: 12368231
-
Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review.
-
Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival.J Neurochem. 2009 May;109 Suppl 1(Suppl 1):133-8. doi: 10.1111/j.1471-4159.2009.05897.x. J Neurochem. 2009. PMID: 19393019 Free PMC article. Review.
Cited by
-
Death by a thousand cuts in Alzheimer's disease: hypoxia--the prodrome.Neurotox Res. 2013 Aug;24(2):216-43. doi: 10.1007/s12640-013-9379-2. Epub 2013 Feb 12. Neurotox Res. 2013. PMID: 23400634 Review.
-
Upregulation of Superoxide Dismutase 2 by Astrocytes in the SIV/Macaque Model of HIV-Associated Neurologic Disease.J Neuropathol Exp Neurol. 2020 Sep 1;79(9):986-997. doi: 10.1093/jnen/nlaa084. J Neuropathol Exp Neurol. 2020. PMID: 32783052 Free PMC article.
-
Acetylated chitosan oligosaccharides act as antagonists against glutamate-induced PC12 cell death via Bcl-2/Bax signal pathway.Mar Drugs. 2015 Mar 12;13(3):1267-89. doi: 10.3390/md13031267. Mar Drugs. 2015. PMID: 25775423 Free PMC article.
-
Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases.Neurochem Res. 2003 Oct;28(10):1563-74. doi: 10.1023/a:1025682611389. Neurochem Res. 2003. PMID: 14570402 Review.
-
Environmental Enrichment Improves Cognitive Deficits, AD Hallmarks and Epigenetic Alterations Presented in 5xFAD Mouse Model.Front Cell Neurosci. 2018 Aug 15;12:224. doi: 10.3389/fncel.2018.00224. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30158856 Free PMC article.
References
-
- Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y. Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke. 1995;26:1478–1489. - PubMed
-
- Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–22940. - PubMed
-
- Chan PH. Oxygen radicals in focal cerebral ischemia. Brain Pathol. 1994;4:59–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
