Differential metabolic activity in the striosome and matrix compartments of the rat striatum during natural behaviors
- PMID: 11756514
- PMCID: PMC6757605
- DOI: 10.1523/JNEUROSCI.22-01-00305.2002
Differential metabolic activity in the striosome and matrix compartments of the rat striatum during natural behaviors
Abstract
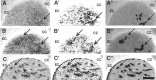
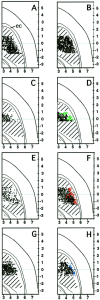
The striosome and matrix compartments of the striatum are clearly identified by their neurochemical expression patterns and anatomical connections. To determine whether these compartments are distinguishable functionally, we used [14C]deoxyglucose metabolic mapping in the rat and tested whether neutral behavioral states (free movement, gentle restraint, and focal tactile stimulation under gentle restraint) were associated with regions of high metabolic activity in the matrix, in striosomes, or in both. We identified metabolic peaks in the striatum by means of image analysis, striosome-matrix boundaries by [3H]naloxone binding, and primary somatosensory corticostriatal input clusters by injections of anterograde tracer into electrophysiologically identified sites in SI. Peak metabolic activity was primarily confined to the matrix compartment under each behavioral condition. These findings show that during relatively neutral behavioral conditions the balance of activity between the two compartments favors the matrix and suggest that this balance is present in the striatum as part of normal behavior and processing of afferent activity.
Figures


Similar articles
-
Preferential localization of self-stimulation sites in striosomes/patches in the rat striatum.Proc Natl Acad Sci U S A. 1998 May 26;95(11):6486-91. doi: 10.1073/pnas.95.11.6486. Proc Natl Acad Sci U S A. 1998. PMID: 9600993 Free PMC article.
-
Role of dynorphin and GABA in the inhibitory regulation of NMDA-induced dopamine release in striosome- and matrix-enriched areas of the rat striatum.J Neurosci. 1994 Apr;14(4):2435-43. doi: 10.1523/JNEUROSCI.14-04-02435.1994. J Neurosci. 1994. PMID: 7908960 Free PMC article.
-
Spatial organization of patch and matrix compartments in the rat striatum.Neuroscience. 1993 Dec;57(3):661-71. doi: 10.1016/0306-4522(93)90013-6. Neuroscience. 1993. PMID: 8309529
-
The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function.ACS Chem Neurosci. 2017 Feb 15;8(2):235-242. doi: 10.1021/acschemneuro.6b00333. Epub 2016 Dec 15. ACS Chem Neurosci. 2017. PMID: 27977131 Review.
-
[Compartmentalized organization of human corpus striatum].Rev Neurol. 1999 Mar 1-15;28(5):512-9. Rev Neurol. 1999. PMID: 10229968 Review. Spanish.
Cited by
-
Integration and propagation of somatosensory responses in the corticostriatal pathway: an intracellular study in vivo.J Physiol. 2011 Jan 15;589(Pt 2):263-81. doi: 10.1113/jphysiol.2010.199646. J Physiol. 2011. PMID: 21059765 Free PMC article.
-
Regulation of motor performance and striatal function by synaptic scaffolding proteins of the Homer1 family.Proc Natl Acad Sci U S A. 2006 Jan 17;103(3):774-9. doi: 10.1073/pnas.0505900103. Epub 2006 Jan 4. Proc Natl Acad Sci U S A. 2006. PMID: 16407107 Free PMC article.
-
Development of repetitive behavior in a mouse model: roles of indirect and striosomal basal ganglia pathways.Int J Dev Neurosci. 2011 Jun;29(4):461-7. doi: 10.1016/j.ijdevneu.2011.02.004. Epub 2011 Feb 15. Int J Dev Neurosci. 2011. PMID: 21329752 Free PMC article.
-
Striatal Cholinergic Interneurons Modulate Spike-Timing in Striosomes and Matrix by an Amphetamine-Sensitive Mechanism.Front Neuroanat. 2017 Mar 21;11:20. doi: 10.3389/fnana.2017.00020. eCollection 2017. Front Neuroanat. 2017. PMID: 28377698 Free PMC article.
-
Subthalamic Nucleus Deep Brain Stimulation Does Not Modify the Functional Deficits or Axonopathy Induced by Nigrostriatal α-Synuclein Overexpression.Sci Rep. 2017 Nov 27;7(1):16356. doi: 10.1038/s41598-017-16690-x. Sci Rep. 2017. PMID: 29180681 Free PMC article.
References
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–272. - PubMed
-
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J Neurophysiol. 1995;73:1234–1252. - PubMed
-
- Bejjani B, Damier P, Arnulf I, Thivard L, Bonnet A, Dormont D, Cornu P, Pidoux B, Samson Y, Agid Y. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476–1480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
