Circadian rhythms in isolated brain regions
- PMID: 11756518
- PMCID: PMC6757616
- DOI: 10.1523/JNEUROSCI.22-01-00350.2002
Circadian rhythms in isolated brain regions
Abstract
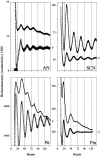
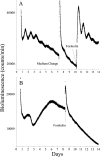
The suprachiasmatic nucleus (SCN) of the mammalian hypothalamus has been referred to as the master circadian pacemaker that drives daily rhythms in behavior and physiology. There is, however, evidence for extra-SCN circadian oscillators. Neural tissues cultured from rats carrying the Per-luciferase transgene were used to monitor the intrinsic Per1 expression patterns in different brain areas and their response to changes in the light cycle. Although many Per-expressing brain areas were arrhythmic in culture, 14 of the 27 areas examined were rhythmic. The pineal and pituitary glands both expressed rhythms that persisted for >3 d in vitro, with peak expression during the subjective night. Nuclei in the olfactory bulb and the ventral hypothalamus expressed rhythmicity with peak expression at night, whereas other brain areas were either weakly rhythmic and peaked at night, or arrhythmic. After a 6 hr advance or delay in the light cycle, the pineal, paraventricular nucleus of the hypothalamus, and arcuate nucleus each adjusted the phase of their rhythmicity with different kinetics. Together, these results indicate that the brain contains multiple, damped circadian oscillators outside the SCN. The phasing of these oscillators to one another may play a critical role in coordinating brain activity and its adjustment to changes in the light cycle.
Figures



References
-
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. - PubMed
-
- Aschoff J, Wever RA. Human circadian rhythms: a multioscillator system. Fed Proc. 1976;35:2326–2332. - PubMed
-
- Aschoff J, Wever RA. Handbook of behavioral neurology. Plenum; New York: 1981. The circadian system of man. pp. 311–331.
-
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. - PubMed
-
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
