Novel mechanism for gonadotropin-releasing hormone neuronal migration involving Gas6/Ark signaling to p38 mitogen-activated protein kinase
- PMID: 11756555
- PMCID: PMC139735
- DOI: 10.1128/MCB.22.2.599-613.2002
Novel mechanism for gonadotropin-releasing hormone neuronal migration involving Gas6/Ark signaling to p38 mitogen-activated protein kinase
Abstract
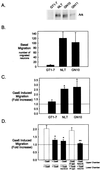
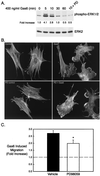
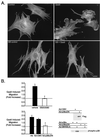
Gonadotropin-releasing hormone (GnRH) is the central regulator of the reproductive axis. Normal sexual maturation depends on the migration of GnRH neurons from the olfactory placode to the hypothalamus during development. Previously, we showed restricted expression of the membrane receptor adhesion-related kinase (Ark) in immortalized cell lines derived from migratory but not postmigratory GnRH neurons. In addition, Ark and GnRH transcripts were detected along the GnRH neuron migratory route in the E13 mouse cribriform plate. In the present study, we examined the role of Ark and its ligand, Gas6 (encoded by growth arrest-specific gene 6), in GnRH neuron migration. Gas6 stimulated lamellipodial extension, membrane ruffling, and chemotaxis of immortalized NLT GnRH neuronal cells via the Ark receptor. Gas6/Ark signaling promoted activation of the Rho family GTPase Rac, and adenoviral-mediated expression of dominant negative N17Rac abolished Gas6/Ark-induced actin cytoskeletal reorganization and migration of GnRH neuronal cells. In addition, p38 MAPK was activated downstream of Ark and Rac, and inhibition of p38 MAPK with either SB203580 or adenoviral dominant negative p38alpha also blocked Gas6/Ark-mediated migration. Finally, downstream of Rac and p38 mitogen-activated protein kinase (MAPK), Gas6/Ark signaling promoted activation of MAPK-activated protein kinase 2 and induced phosphorylation of HSP25, a known regulator of cortical actin remodeling. The data are the first to demonstrate a migratory signaling pathway downstream of Ark/Axl family receptors and suggest a previously unidentified role for p38 MAPK in neuronal migration. Furthermore, these studies support a potential role for Ark in the regulation of GnRH neuronal migration.
Figures




References
-
- Allen, M. P., M. Xu, C. Zeng, S. A. Tobet, and M. E. Wierman. 2000. Myocyte enhancer factors-2B and -2C are required for adhesion related kinase repression of neuronal gonadotropin releasing hormone gene expression. J. Biol. Chem. 275:39662–39670. - PubMed
-
- Allen, M. P., C. Zeng, K. Schneider, X. Xiong, M. K. Meintzer, P. Bellosta, C. Basilico, B. Varnum, K. A. Heidenreich, and M. E. Wierman. 1999. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol. Endocrinol. 13:191–201. - PubMed
-
- Bateman, J., H. Shu, and D. Van Vactor. 2000. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron 26:93–106. - PubMed
-
- Bellosta, P., Q. Zhang, S. P. Goff, and C. Basilico. 1997. Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene 15:2387–2397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
