Hpt, a bacterial homolog of the microsomal glucose- 6-phosphate translocase, mediates rapid intracellular proliferation in Listeria
- PMID: 11756655
- PMCID: PMC117577
- DOI: 10.1073/pnas.012363899
Hpt, a bacterial homolog of the microsomal glucose- 6-phosphate translocase, mediates rapid intracellular proliferation in Listeria
Abstract
Efficient replication in vivo is essential for a microparasite to colonize its host and the understanding of the molecular mechanisms by which microbial pathogens grow within host tissues can lead to the discovery of novel therapies to treat infection. Here we present evidence that the foodborne bacterial pathogen Listeria monocytogenes, a facultative intracellular parasite, exploits hexose phosphates (HP) from the host cell as a source of carbon and energy to fuel fast intracellular growth. HP uptake is mediated by Hpt, a bacterial homolog of the mammalian translocase that transports glucose-6-phosphate from the cytosol into the endoplasmic reticulum in the final step of gluconeogenesis and glycogenolysis. Expression of the Hpt permease is tightly controlled by the central virulence regulator PrfA, which upon entry into host cells induces a set of virulence factors required for listerial intracellular parasitism. Loss of Hpt resulted in impaired listerial intracytosolic proliferation and attenuated virulence in mice. Hpt is the first virulence factor to be identified as specifically involved in the replication phase of a facultative intracellular pathogen. It is also a clear example of how adaptation to intracellular parasitism by microbial pathogens involves mimicry of physiological mechanisms of their eukaryotic host cells.
Figures


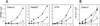

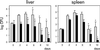

References
-
- Cossart P, Portnoy D A. In: Gram-Positive Pathogens. Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Washington, DC: Am. Soc. Microbiol.; 2000. pp. 507–515.
-
- Goebel W, Kreft J, Böckmann R. In: Gram-Positive Pathogens. Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Washington, DC: Am. Soc. Microbiol.; 2000. pp. 499–506.
-
- Kreft J, Vázquez-Boland J A. Int J Med Microbiol Infect Dis. 2001;291:145–157. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

