Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response
- PMID: 11756671
- PMCID: PMC117563
- DOI: 10.1073/pnas.012511599
Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response
Abstract
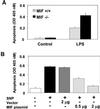
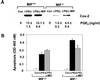
The importance of the macrophage in innate immunity is underscored by its secretion of an array of powerful immunoregulatory and effector molecules. We report herein that macrophage migration inhibitory factor (MIF), a product of activated macrophages, sustains macrophage survival and function by suppressing activation-induced, p53-dependent apoptosis. Endotoxin administration to MIF(-/-) mice results in decreased macrophage viability, decreased proinflammatory function, and increased apoptosis when compared with wild-type controls. Moreover, inhibition of p53 in endotoxin-treated, MIF-deficient macrophages suppresses enhanced apoptosis and restores proinflammatory function. MIF inhibits p53 activity in macrophages via an autocrine regulatory pathway, resulting in a decrease in cellular p53 accumulation and subsequent function. Inhibition of p53 by MIF coincides with the induction of arachidonic acid metabolism and cyclooxygenase-2 (Cox-2) expression, which is required for MIF regulation of p53. MIF's effect on macrophage viability and survival provides a previously unrecognized mechanism to explain its critical proinflammatory action in conditions such as sepsis, and suggests new approaches for the modulation of innate immune responses.
Figures




References
-
- Hoffmann J A, Kafatos F C, Janeway C A, Ezekowitz R A. Science. 1999;284:1313–1318. - PubMed
-
- Dinarello C A. Chest. 2000;118:503–508. - PubMed
-
- Ohmori Y, Hamilton T A. Pharmacol Ther. 1994;63:235–264. - PubMed
-
- Albina J E, Cui S, Mateo R B, Reichner J S. J Immunol. 1993;150:5080–5085. - PubMed
-
- Sarih M, Souvannavong V, Adam A. Biochem Biophys Res Commun. 1993;191:503–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

