Structure determination of micelle-like intermediates in amyloid beta -protein fibril assembly by using small angle neutron scattering
- PMID: 11756677
- PMCID: PMC117530
- DOI: 10.1073/pnas.012584899
Structure determination of micelle-like intermediates in amyloid beta -protein fibril assembly by using small angle neutron scattering
Abstract
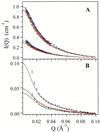
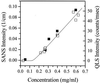
Increasing evidence supports the hypothesis that amyloid beta-protein (Abeta) assembly is a key pathogenic feature of Alzheimer's disease. Thus, understanding the assembly process offers opportunities for the development of strategies for treating this devastating disease. In prior studies, Abeta was found to form micelle-like aggregates under acidic conditions. These structures exhibited an average observed hydrodynamic radius of 7 nm. They were found to be in rapid equilibrium with Abeta monomers or low molecular weight oligomers, and were centers of fibril nucleation. Here the technique of small angle neutron scattering has been used to determine the structure of these Abeta micelles. The data reveal that the micellar assemblies comprise 30-50 Abeta monomers and have elongated geometries. The best fit of the data to a uniform spherocylinder yields a radius approximately 2.4 nm and cylinder length approximately 11 nm. These structure parameters remain constant over more than a decade in concentration range. The concentration independence of the length of the cylindrical aggregate indicates the presence of an internal nonrepetitive structure that spans the entire length of the Abeta assembly.
Figures



Similar articles
-
Micellization of surfactin and its effect on the aggregate conformation of amyloid beta(1-40).J Phys Chem B. 2008 Nov 27;112(47):15195-201. doi: 10.1021/jp805966x. J Phys Chem B. 2008. PMID: 18983185
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
-
On the nucleation and growth of amyloid beta-protein fibrils: detection of nuclei and quantitation of rate constants.Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1125-9. doi: 10.1073/pnas.93.3.1125. Proc Natl Acad Sci U S A. 1996. PMID: 8577726 Free PMC article.
-
Membrane charge dependent states of the beta-amyloid fragment Abeta (16-35) with differently charged micelle aggregates.Biochim Biophys Acta. 2010 Mar;1798(3):660-71. doi: 10.1016/j.bbamem.2009.12.012. Epub 2010 Jan 4. Biochim Biophys Acta. 2010. PMID: 20045392
-
Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer's disease.Neurol Res. 2005 Dec;27(8):869-81. doi: 10.1179/016164105X49436. Neurol Res. 2005. PMID: 16354549 Review.
Cited by
-
Hsp20, a novel alpha-crystallin, prevents Abeta fibril formation and toxicity.Protein Sci. 2005 Mar;14(3):593-601. doi: 10.1110/ps.041020705. Protein Sci. 2005. PMID: 15722443 Free PMC article.
-
Overview of Alzheimer's Disease and Some Therapeutic Approaches Targeting Aβ by Using Several Synthetic and Herbal Compounds.Oxid Med Cell Longev. 2016;2016:7361613. doi: 10.1155/2016/7361613. Epub 2015 Dec 28. Oxid Med Cell Longev. 2016. PMID: 27034741 Free PMC article. Review.
-
Molecular dynamics simulation of amyloid beta dimer formation.Biophys J. 2004 Oct;87(4):2310-21. doi: 10.1529/biophysj.104.040980. Biophys J. 2004. PMID: 15454432 Free PMC article.
-
Anatomy and formation mechanisms of early amyloid-β oligomers with lateral branching: graph network analysis on large-scale simulations.Chem Sci. 2022 Feb 8;13(9):2649-2660. doi: 10.1039/d1sc06337e. eCollection 2022 Mar 2. Chem Sci. 2022. PMID: 35356670 Free PMC article.
-
Fatty Acid Concentration and Phase Transitions Modulate Aβ Aggregation Pathways.Sci Rep. 2017 Sep 4;7(1):10370. doi: 10.1038/s41598-017-09794-x. Sci Rep. 2017. PMID: 28871093 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

