Quaternary structure of human fatty acid synthase by electron cryomicroscopy
- PMID: 11756679
- PMCID: PMC117528
- DOI: 10.1073/pnas.012589499
Quaternary structure of human fatty acid synthase by electron cryomicroscopy
Abstract
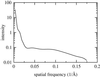
We present the first three-dimensional reconstruction of human fatty acid synthase obtained by electron cryomicroscopy and single-particle image processing. The structure shows that the synthase is composed of two monomers, arranged in an antiparallel orientation, which is consistent with biochemical data. The monomers are connected to each other at their middle by a bridge of density, a site proposed to be the combination of the interdomain regions of the two monomers. Each monomer subunit appears to be subdivided into three structural domains. With this reconstruction of the synthase, we propose a location for the enzyme's two fatty acid synthesis sites.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

