How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis
- PMID: 11762571
- PMCID: PMC81663
How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis
Abstract
Background: Numerous small clinical trials have been carried out to study the behaviourally defined efficacy and safety of short-acting methylphenidate compared with placebo for attention-deficit disorder (ADD) in individuals aged 18 years and less. However, no meta-analyses that carefully examined these questions have been done. We reviewed the behavioural evidence from all the randomized controlled trials that compared methylphenidate and placebo, and completed a meta-analysis.
Methods: We searched several electronic sources for articles published between 1981 and 1999: MEDLINE, EMBASE, PsychINFO, ERIC, CINAHL, HEALTHSTAR, Biological Abstracts, Current Contents and Dissertation Abstracts. The Cochrane Library Trials Registry and Current Controlled Trials were also consulted. A study was considered eligible for inclusion if it entailed the following: a placebo-controlled randomized trial that involved short-acting methylphenidate and participants aged 18 years or less at the start of the trial who had received any primary diagnosis of ADD that was made in a systematic and reproducible way.
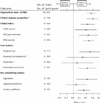
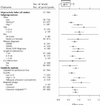
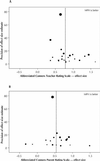
Results: We included 62 randomized trials that involved a total of 2897 participants with a primary diagnosis of ADD (e.g., with or without hyperactivity). The median age of trial participants was 8.7 years, and the median "percent male" composition of trials was 88.1%. Most studies used a crossover design. Using the scores from 2 separate indices, this collection of trials exhibited low quality. Interventions lasted, on average, 3 weeks, with no trial lasting longer than 28 weeks. Each primary outcome (hyperactivity index) demonstrated a significant effect of methylphenidate (effect size reported by teacher 0.78, 95% confidence interval [CI] 0.64-0.91; effect size reported by parent 0.54, 95% CI 0.40-0.67). However, these apparent beneficial effects are tempered by a strong indication of publication bias and the lack of robustness of the findings, especially those involving core ADD features. Methylphenidate also has an adverse event profile that requires consideration. For example, clinicians only need to treat 4 children to identify an episode of decreased appetite.
Interpretation: Short-acting methylphenidate has a statistically significant clinical effect in the short-term treatment of individuals with a diagnosis of ADD aged 18 years and less. However, the extension of this placebo-controlled effect beyond 4 weeks of treatment has not been demonstrated. Exact knowledge of the extent and definition of the short-term behavioural usefulness of methylphenidate is questioned.
Figures


Comment in
-
Methylphenidate in the treatment of children with attention-deficit hyperactivity disorder.CMAJ. 2001 Nov 27;165(11):1505-6. CMAJ. 2001. PMID: 11762576 Free PMC article. Review. No abstract available.
-
Review: short acting methylphenidate has short term efficacy in children and adolescents with attention deficit disorder.Evid Based Ment Health. 2002 May;5(2):50. doi: 10.1136/ebmh.5.2.50. Evid Based Ment Health. 2002. PMID: 12026899 No abstract available.
-
Treatment of attention-deficit hyperactivity disorder.CMAJ. 2002 May 14;166(10):1251-2. CMAJ. 2002. PMID: 12041836 Free PMC article. No abstract available.
References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). Washington: The Association; 1994.
-
- Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of Attention-Deficit/Hyperactivity Disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA 1998; 279: 1100-7. - PubMed
-
- Jadad AR, Boyle M, Cunningham C, Kim M, Schachar R. Treatment of Attention-Deficit/Hyperactivity Disorder. Prepared for Agency of Healthcare Research and Quality. Rockville (MD): The Agency; 1999.
-
- Elia J, Ambrosini P, Rapoport J. Treatment of Attention-Deficit/Hyperactivity Disorder. N Engl J Med 1999;340:780-8. - PubMed
-
- Barkley RA, editor. Attention-deficit hyperactivity disorder. A handbook for diagnosis and treatment. 2nd ed. New York: The Guilford Press; 1998.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
