Toxicokinetics of tremorogenic natural products, harmane and harmine, in male Sprague-Dawley rats
- PMID: 11766171
- PMCID: PMC4992346
- DOI: 10.1080/152873901753246241
Toxicokinetics of tremorogenic natural products, harmane and harmine, in male Sprague-Dawley rats
Abstract
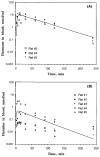
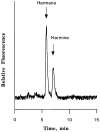
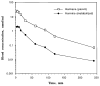
Tremorogenic beta-carboline alkaloids are present in foodstuffs and beverages. Acute exposure to beta-carboline derivatives causes severe tremor; however, the disposition of these dietary contaminants remains unclear. This study was performed to evaluate toxicokinetics of harmane and harmine, two major beta-carboline alkaloids, in rats. Blood concentrations of both toxicants were quantified by high-performance liquid chromatography (HPLC). Following an intravenous injection (0.5 mg/kg), the concentration-time profiles of harmane or harmine fit well with a two-compartment model. While both compounds had comparable elimination t 1/2beta (24 and 26 min for harmane and harmine, respectively), the systemic clearance (CLs) for harmine (103.2 ml/kg/ml) was two times greater than that for harmane (52.2 ml/kg/ml). Accordingly, the area under the blood concentration-time curve (AUC) in harmane-treated rats was 2.7-fold greater than that in harmine-treated rats. Harmine appeared to distribute to tissues better than harmane, with a larger volume of distribution (V,d) (3.9 and 1.6 L/kg for harmine and harmane, respectively). After an oral dose (20 mg/kg), the absolute bioavailability (F) was 19% for harmane and 3% for harmine. Harmane was absorbed more slowly (lower Ka), yet more completely (higher Cmax' AUC, and F) than harmine. An oral administration of harmane resulted in blood harmine whose formation accounted for 13% of the ingested harmane, indicating a biotransformation of harmane to harmine. These results suggest that harmane is absorbed into the systemic circulation more completely than harmine. Upon entering the body, harmane can be metabolized to form harmine; the latter may better distribute to the tissue compartment.
Figures


References
-
- Adachi J, Mizoi Y, Naito T, Yamamoto K, Fujiwara S, Ninomiya I. Determination of β-carbolines in foodstuffs by high performance liquid chromatography and high performance liquid chromatograph-mass spectrometry. J Chromatogr. 1991;538:331–339. - PubMed
-
- Allen RF, Beck O, Borg S, Skroder R. Analysis of 1-methyl-1,2,3,4-tetrahydro-β-carboline in human urine and cerebrospinal fluid by gas chromatography–mass spectrometry. Eur J Mass Spectrom. 1980;1:171–177.
-
- Bidder TA, Shoemaker DW, Boettger HG, Evans M, Cummins JT. Harmane in human platelets. Life Sci. 1979;25:157–164. - PubMed
-
- Du W, Aloyo VJ, Harvey JA. Harmaline competitively inhibits [3H]MK-801 binding to the NMDA receptor in rabbit brain. Brain Res. 1997;770:26–29. - PubMed
-
- Fuentes LA, Longo VG. An investigation on the central effects of harmine, harmaline and related B-carbolines. Neuropharmacology. 1971;10:15–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
