Hyperthermostable endoglucanase from Pyrococcus horikoshii
- PMID: 11772658
- PMCID: PMC126571
- DOI: 10.1128/AEM.68.1.430-433.2002
Hyperthermostable endoglucanase from Pyrococcus horikoshii
Abstract
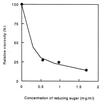
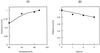
An endoglucanase homolog from the hyperthermophilic archaeon Pyrococcus horikoshii was expressed in Escherichia coli, and its enzymatic characteristics were examined. The expressed protein was a hyperthermostable endoglucanase which hydrolyzes celluloses, including Avicel and carboxymethyl cellulose, as well as beta-glucose oligomers. This enzyme is the first endoglucanase belonging to glycosidase family 5 found from Pyrococcus species and is also the first hyperthermostable endoglucanase to which celluloses are the best substrates. This enzyme is expected to be useful for industrial hydrolysis of cellulose at high temperatures, particularly in biopolishing of cotton products.
Figures

References
-
- Ando, S., K. Ishikawa, H. Ishida, Y. Kawarabayasi, H. Kikuchi, and Y. Kosugi. 1999. Thermostable aminopeptidase from Pyrococcus horikoshii. FEBS Lett. 447:25–28. - PubMed
-
- Gilkes, N. R., M. L. Langsford, D. G. Kilburn, R. C. Miller, Jr., and R. A. J. Warren. 1984. Mode of action and substrate specificities of cellulases from cloned bacterial genes. J. Biol. Chem. 259:10455–10459. - PubMed
-
- Gonzalez, J. M., Y. Masuchi, F. T. Robb, J. W. Ammerman, D. L. Maeder, M. Yanagibayashi, J. Tamaoka, and C. Kato. 1998. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles 2:123–130. - PubMed
-
- Gueguen, Y., W. G. B. Voorhorst, J. van der Oost, and W. M. de Vos. 1997. Molecular and biochemical characterization of an endo-β1-3-glucanase of the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 272:31258–31264. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

