A human RNA polymerase II-containing complex associated with factors necessary for spliceosome assembly
- PMID: 11773074
- PMCID: PMC4492735
- DOI: 10.1074/jbc.M110516200
A human RNA polymerase II-containing complex associated with factors necessary for spliceosome assembly
Abstract
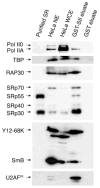
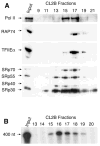
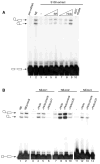
Transcription and splicing are coordinated processes in mammalian cells. We have used affinity chromatography with immobilized transcription elongation factor SII to purify a protein complex that contains core RNA polymerase II (RNA Pol II), the general transcription initiation factors, and several splicing factors, including the U1, U2, and U4 small nuclear RNPs, the U2AF(65), and serine/arginine-rich proteins. The splicing factors and the transcription machinery co-purify through a gel filtration column and co-immunoprecipitate in experiments using an anti-U2AF(65) antibody, indicating that they are part of a unique complex. Although the RNA Pol II-containing complex does not possess splicing activity, it can complement small nuclear RNP-inactivated extracts and can promote the formation of a pre-spliceosome complex. Because interactions between components of the splicing and transcription machineries occur in the context of a complex containing a hypophosphorylated RNA Pol II capable of initiating transcription, our results suggest that the coupling between transcription and splicing begins before transcription initiation.
Figures



References
-
- Kramer A. Annu Rev Biochem. 1996;65:367–409. - PubMed
-
- Moore MJ, Query CC, Sharp PA. In: The RNA World: the Nature of Modern RNA Suggests a Prebiotic RNA World. Gesteland RF, Atkins JF, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. pp. 303–358.
-
- Bauren G, Wieslander L. Cell. 1994;76:183–192. - PubMed
-
- Beyer AL, Yvonne N, Osheim YN. Genes Dev. 1988;2:754–765. - PubMed
-
- Misteli T, Spector DL. Mol Cell. 1999;3:697–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

