Modulation of network behaviour by changes in variance in interneuronal properties
- PMID: 11773331
- PMCID: PMC2290026
- DOI: 10.1113/jphysiol.2001.013054
Modulation of network behaviour by changes in variance in interneuronal properties
Abstract
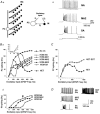
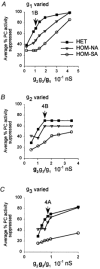
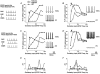
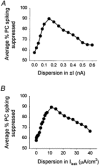
Interneurones are important regulators of neuronal networks. The conventional approach to interneurones is to focus on the mean values of various parameters. Here we tested the hypothesis that changes in the variance of interneuronal properties (e.g. in the degree of scattering of parameter values of individual cells around the population mean) may modify the behaviour of networks. Biophysically based multicompartmental models of principal cells and interneurones showed that changes in the variance in the electrophysiological and anatomical properties of interneurones significantly alter the input-output functions, rhythmicity and synchrony of principal cells, even if the mean values were unchanged. In most cases, increased heterogeneity in interneurones resulted in stronger inhibition of principal cell firing; however, there were parameter ranges where increased interneuronal variance decreased the inhibition of principal cells. Electrophysiological recordings showed that the variance in the resting membrane potential of CA1 stratum oriens interneurones persistently increased following experimental complex febrile seizures in developing rats, without a change in the mean resting membrane potential, indicating that lasting alterations in interneuronal heterogeneity can take place in real neuronal systems. These computational and experimental data demonstrate that modifications in interneuronal population variance influence the behaviour of neuronal networks, and suggest a physiological role for interneuronal diversity. Furthermore, the results indicate that interneuronal heterogeneity can change in neurological diseases, and raise the possibility that neuromodulators may act by regulating the variance of key parameters in interneuronal populations.
Figures








References
-
- Ali AB, Bannister AP, Thomson AM. IPSPs elicited in CA1 pyramidal cells by putative basket cells in slices of adult rat hippocampus. European Journal of Neuroscience. 1999;11:1741–1753. - PubMed
-
- Aradi I, Holmes WR. Active dendrites regulate spatio-temporal synaptic integration in hippocampal dentate granule cells. Neurocomputing. 1999a;26:45–51.
-
- Aradi I, Holmes WR. Role of multiple calcium and calcium-dependent conductances in regulation of hippocampal dentate granule cell excitability. Journal of Computational Neuroscience. 1999b;6:215–235. - PubMed
-
- Banks MI, White JA, Pearce RA. Interactions between distinct GABAA circuits in hippocampus. Neuron. 2000;25:449–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

