Human cytomegalovirus UL47 tegument protein functions after entry and before immediate-early gene expression
- PMID: 11773380
- PMCID: PMC135821
- DOI: 10.1128/jvi.76.3.1043-1050.2002
Human cytomegalovirus UL47 tegument protein functions after entry and before immediate-early gene expression
Abstract
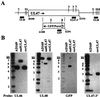
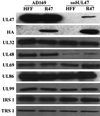
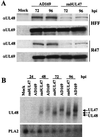
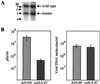
The human cytomegalovirus UL47 open reading frame encodes a 110-kDa protein that is a component of the virion tegument. We have constructed a cytomegalovirus mutant, ADsubUL47, in which the central portion of the UL47 open reading frame has been replaced by two marker genes. The mutant replicated to titers 100-fold lower than those for wild-type virus after infection at either a high or a low input multiplicity in primary human fibroblasts but was substantially complemented on cells expressing UL47 protein. A revertant virus in which the mutation was repaired, ADrevUL47, replicated with wild-type kinetics. Mutant virions lacked UL47 protein and contained reduced amounts of UL48 protein. The mutant was found to be less infectious than wild-type virus, and a defect very early in the replication cycle was observed. Transcription of the viral immediate-early 1 gene was delayed by 8 to 10 h. However, this delay was not the result of a defect in virus entry or of the inability of virion proteins to transactivate the major immediate-early promoter. We also show that the UL47 protein coprecipitated with the UL48 and UL69 tegument proteins and the UL86-encoded major capsid protein. We propose that a UL47-containing complex is involved in the release of viral DNA from the disassembling virus particle and that the loss of UL47 protein causes this process to be delayed.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

