Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone
- PMID: 11773385
- PMCID: PMC135799
- DOI: 10.1128/jvi.76.3.1089-1099.2002
Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone
Abstract
Reverse genetics was used to develop a two-component, trivalent live attenuated vaccine against human parainfluenza virus type 3 (HPIV3) and respiratory syncytial virus (RSV) subgroups A and B. The backbone for each of the two components of this vaccine was the attenuated recombinant bovine/human PIV3 (rB/HPIV3), a recombinant BPIV3 in which the bovine HN and F protective antigens are replaced by their HPIV3 counterparts (48). This chimera retains the well-characterized host range attenuation phenotype of BPIV3, which appears to be appropriate for immunization of young infants. The open reading frames (ORFs) for the G and F major protective antigens of RSV subgroup A and B were each placed under the control of PIV3 transcription signals and inserted individually or in homologous pairs as supernumerary genes in the promoter proximal position of rB/HPIV3. The level of replication of rB/HPIV3-RSV chimeric viruses in the respiratory tract of rhesus monkeys was similar to that of their parent virus rB/HPIV3, and each of the chimeras induced a robust immune response to both RSV and HPIV3. RSV-neutralizing antibody titers induced by rB/HPIV3-RSV chimeric viruses were equivalent to those induced by infection with wild-type RSV, and HPIV3-specific antibody responses were similar to, or slightly less than, after infection with the rB/HPIV3 vector itself. This study describes a novel vaccine strategy against RSV in which vaccine viruses with a common attenuated backbone, specifically rB/HPIV3 derivatives expressing the G and/or F major protective antigens of RSV subgroup A and of RSV subgroup B, are used to immunize by the intranasal route against RSV and HPIV3, which are the first and second most important viral agents of pediatric respiratory tract disease worldwide.
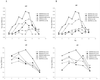
Figures


References
-
- Bailly, J. E., J. M. McAuliffe, M. H. Skiadopoulos, P. L. Collins, and B. R. Murphy. 2000. Sequence determination and molecular analysis of two strains of bovine parainfluenza virus type 3 that are attenuated for primates. Virus Genes 20:173–182. - PubMed
-
- Belshe, R. B., L. S. Richardson, W. T. London, D. L. Sly, J. H. Lorfeld, E. Camargo, D. A. Prevar, and R. M. Chanock. 1977. Experimental respiratory syncytial virus infection of four species of primates. J. Med. Virol. 1:157–162. - PubMed
-
- Buchholz, U. J., H. Granzow, K. Schuldt, S. S. Whitehead, B. R. Murphy, and P. L. Collins. 2000. Chimeric bovine respiratory syncytial virus with glycoprotein gene substitutions from human respiratory syncytial virus (HRSV): effects on host range and evaluation as a live-attenuated HRSV vaccine. J. Virol. 74:1187–1199. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

