Transcription regulatory sequences and mRNA expression levels in the coronavirus transmissible gastroenteritis virus
- PMID: 11773405
- PMCID: PMC135778
- DOI: 10.1128/jvi.76.3.1293-1308.2002
Transcription regulatory sequences and mRNA expression levels in the coronavirus transmissible gastroenteritis virus
Abstract
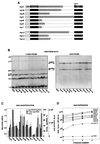
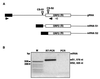
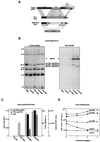
The transcription regulatory sequences (TRSs) of the coronavirus transmissible gastroenteritis virus (TGEV) have been characterized by using a helper virus-dependent expression system based on coronavirus-derived minigenomes to study the synthesis of subgenomic mRNAs. The TRSs are located at the 5' end of TGEV genes and include a highly conserved core sequence (CS), 5'-CUAAAC-3', that is essential for mediating a 100- to 1,000-fold increase in mRNA synthesis when it is located in the appropriate context. The relevant sequences contributing to TRS activity have been studied by extending the CS 5' upstream and 3' downstream. Sequences from virus genes flanking the CS influenced transcription levels from moderate (10- to 20-fold variation) to complete mRNA synthesis silencing, as shown for a canonical CS at nucleotide (nt) 120 from the initiation codon of the S gene that did not lead to the production of the corresponding mRNA. An optimized TRS has been designed comprising 88 nt from the N gene TRS, the CS, and 3 nt 3' to the M gene CS. Further extension of the 5'-flanking nucleotides (i.e., by 176 nt) decreased subgenomic RNA levels. The expression of a reporter gene (beta-glucuronidase) by using the selected TRS led to the production of 2 to 8 microg of protein per 10(6) cells. The presence of an appropriate Kozak context led to a higher level of protein expression. Virus protein levels were shown to be dependent on transcription and translation regulation.
Figures


References
-
- Alonso, S., I. Sola, H. Wege, J. Teifke, M. Balach, J. Plana-Durán, and L. Enjuanes. Heterologous gene expression in tissue culture and in vivo using a transmissible gastroenteritis coronavirus helper dependent system. J. Gen. Virol., in press.
-
- Bronstein, I., J. J. Fortin, J. C. Voyta, R.-R. Juo, B. Edwards, C. E. M. Olenses, N. Lijam, and L. J. Kricka. 1994. Chemiluminescent reporter gene assays: sensitive detection of the GUS and SEAP gene products. BioTechniques 17:172–177. - PubMed
-
- Enjuanes, L., D. Brian, D. Cavanagh, K. Holmes, M. M. C. Lai, H. Laude, P. Masters, P. Rottier, S. G. Siddell, W. J. M. Spaan, F. Taguchi, and P. Talbot. 2000. Coronaviridae, p.835–849. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carsten, M. K. Estes, S. M. Lemon, D. J. McGeoch, J. Maniloff, M. A. Mayo, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Classification and nomenclature of viruses. Academic Press, Inc., New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

