Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein
- PMID: 11773406
- PMCID: PMC135838
- DOI: 10.1128/jvi.76.3.1309-1327.2002
Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein
Abstract
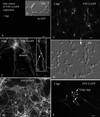
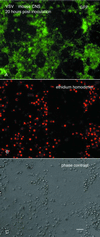
A new recombinant vesicular stomatitis virus (rVSV) that expresses green fluorescent protein (GFP) on the cytoplasmic domain of the VSV glycoprotein (G protein) was used in the mouse as a model for studying brain infections by a member of the Mononegavirales order that can cause permanent changes in behavior. After nasal administration, virus moved down the olfactory nerve, first to periglomerular cells, then past the mitral cell layer to granule cells, and finally to the subventricular zone. Eight days postinoculation, rVSV was eliminated from the olfactory bulb. Little sign of infection could be found outside the olfactory system, suggesting that anterograde or retrograde axonal transport of rVSV was an unlikely mechanism for movement of rVSV out of the bulb. When administered intracerebrally by microinjection, rVSV spread rapidly within the brain, with strong infection at the site of injection and at some specific periventricular regions of the brain, including the dorsal raphe, locus coeruleus, and midline thalamus; the ventricular system may play a key role in rapid rVSV dispersion within the brain. Thus, the lack of VSV movement out of the olfactory system was not due to the absence of potential for infections in other brain regions. In cultures of both mouse and human central nervous system (CNS) cells, rVSV inoculations resulted in productive infection, expression of the G-GFP fusion protein in the dendritic and somatic plasma membrane, and death of all neurons and glia, as detected by ethidium homodimer nuclear staining. Although considered a neurotropic virus, rVSV also infected heart, skin, and kidney cells in dispersed cultures. rVSV showed a preference for immature neurons in vitro, as shown by enhanced viral infection in developing hippocampal cultures and in the outer granule cell layer in slices of developing cerebellum. Together, these data suggest a relative affinity of rVSV for some neuronal types in the CNS, adding to our understanding of the long-lasting changes in rodent behavior found after transient VSV infection.
Figures




Similar articles
-
Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis.Brain Res. 1994 Jan 28;635(1-2):81-95. doi: 10.1016/0006-8993(94)91426-5. Brain Res. 1994. PMID: 8173982
-
Upon intranasal vesicular stomatitis virus infection, astrocytes in the olfactory bulb are important interferon Beta producers that protect from lethal encephalitis.J Virol. 2015 Mar;89(5):2731-8. doi: 10.1128/JVI.02044-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540366 Free PMC article.
-
Mouse cytomegalovirus in developing brain tissue: analysis of 11 species with GFP-expressing recombinant virus.J Comp Neurol. 2000 Nov 27;427(4):559-80. doi: 10.1002/1096-9861(20001127)427:4<559::aid-cne5>3.0.co;2-4. J Comp Neurol. 2000. PMID: 11056464
-
Host strategies against virus entry via the olfactory system.Virulence. 2011 Jul-Aug;2(4):367-70. doi: 10.4161/viru.2.4.16138. Epub 2011 Jul 1. Virulence. 2011. PMID: 21758005 Review.
-
Viruses and behavioural changes: a review of clinical and experimental findings.Rev Neurosci. 1993 Jul-Sep;4(3):267-86. doi: 10.1515/revneuro.1993.4.3.267. Rev Neurosci. 1993. PMID: 9155866 Review.
Cited by
-
Enhanced cytomegalovirus infection of developing brain independent of the adaptive immune system.J Virol. 2002 Sep;76(17):8842-54. doi: 10.1128/jvi.76.17.8842-8854.2002. J Virol. 2002. PMID: 12163604 Free PMC article.
-
Replication and propagation of attenuated vesicular stomatitis virus vectors in vivo: vector spread correlates with induction of immune responses and persistence of genomic RNA.J Virol. 2007 Feb;81(4):2078-82. doi: 10.1128/JVI.02525-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151110 Free PMC article.
-
A VSV-based Zika virus vaccine protects mice from lethal challenge.Sci Rep. 2018 Jul 23;8(1):11043. doi: 10.1038/s41598-018-29401-x. Sci Rep. 2018. PMID: 30038228 Free PMC article.
-
Neurovirulence and immunogenicity of attenuated recombinant vesicular stomatitis viruses in nonhuman primates.J Virol. 2014 Jun;88(12):6690-701. doi: 10.1128/JVI.03441-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696472 Free PMC article.
-
Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism.J Virol. 2011 Jun;85(11):5679-84. doi: 10.1128/JVI.02511-10. Epub 2011 Mar 30. J Virol. 2011. PMID: 21450833 Free PMC article.
References
-
- Balachandran, S., and G. N. Barber. 2000. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 50:135–138. - PubMed
-
- Card, J. P., M. E. Whealy, A. K. Robbins, R. Y. Moore, and L. W. Enquist. 1991. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron 6:957–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

