The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway
- PMID: 11773417
- PMCID: PMC135835
- DOI: 10.1128/jvi.76.3.1435-1449.2002
The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway
Abstract
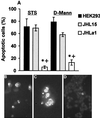
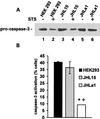
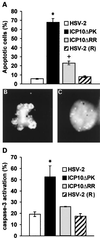
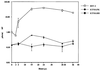
Herpes simplex virus type 1 (HSV-1) and HSV-2 trigger or counteract apoptosis by a cell-specific mechanism. Our studies are based on previous findings that the protein kinase (PK) domain of the large subunit of HSV-2 ribonucleotide reductase (ICP10) activates the Ras/MEK/MAPK pathway (Smith et al., J. Virol. 74:10417, 2000). Because survival pathways can modulate apoptosis, we used cells that are stably or transiently transfected with ICP10 PK, an HSV-2 mutant deleted in ICP10 PK (ICP10DeltaPK) and the MEK-specific inhibitor U0126 to examine the role of ICP10 PK in apoptosis. Apoptosis was induced by staurosporine or D-mannitol in human (HEK293) cells or HEK293 cells stably transfected with the ICP10 PK-negative mutant p139 (JHL15), as determined by morphology, DNA fragmentation, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL), caspase-3 activation, and poly(ADP-ribose) polymerase (PARP) cleavage. HEK293 cells stably transfected with ICP10 (JHLa1) were protected from apoptosis. ICP10 but not p139 protected neuronally differentiated PC12 cells from death due to nerve growth factor withdrawal, and apoptosis (determined by TUNEL) and caspase-3 activation were seen in primary hippocampal cultures infected with ICP10DeltaPK but not with HSV-2 or a revertant virus [HSV-2(R)]. The data indicate that ICP10 has antiapoptotic activity under both paradigms and that it requires a functional PK activity. The apoptotic cells in primary hippocampal cultures were neurons, as determined by double immunofluorescence with fluorescein-labeled dUTP (TUNEL) and phycoerythrin-labeled antibodies specific for neuronal proteins (TuJ1 and NF-160). Protection from apoptosis was associated with MEK/MAPK activation, as evidenced by (i) increased levels of activated (phosphorylated) MAPK in HSV-2- but not ICP10DeltaPK-infected cultures and (ii) inhibition of MAPK activation by the MEK-specific inhibitor U0126. MEK and MAPK were activated by infection with UV-inactivated but not antibody-neutralized HSV-2, suggesting that activation requires cellular penetration but is independent of de novo viral protein synthesis.
Figures







Similar articles
-
The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) functions as a dominant regulator of apoptosis in hippocampal neurons involving activation of the ERK survival pathway and upregulation of the antiapoptotic protein Bag-1.J Virol. 2003 Jan;77(2):1292-305. doi: 10.1128/jvi.77.2.1292-1305.2003. J Virol. 2003. PMID: 12502846 Free PMC article.
-
Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth.J Virol. 2000 Nov;74(22):10417-29. doi: 10.1128/jvi.74.22.10417-10429.2000. J Virol. 2000. PMID: 11044086 Free PMC article.
-
Growth-compromised HSV-2 vector Delta RR protects from N-methyl-D-aspartate-induced neuronal degeneration through redundant activation of the MEK/ERK and PI3-K/Akt survival pathways, either one of which overrides apoptotic cascades.J Neurosci Res. 2008 Feb 1;86(2):378-91. doi: 10.1002/jnr.21486. J Neurosci Res. 2008. PMID: 17893911
-
The herpes simplex virus type 2 protein ICP10PK: a master of versatility.Front Biosci. 2005 Sep 1;10:2820-31. doi: 10.2741/1738. Front Biosci. 2005. PMID: 15970536 Review.
-
Targeting apoptosis in neurological disease using the herpes simplex virus.J Cell Mol Med. 2002 Jul-Sep;6(3):341-56. doi: 10.1111/j.1582-4934.2002.tb00513.x. J Cell Mol Med. 2002. PMID: 12417051 Free PMC article. Review.
Cited by
-
Intranasal administration of the growth-compromised HSV-2 vector DeltaRR prevents kainate-induced seizures and neuronal loss in rats and mice.Mol Ther. 2006 May;13(5):870-81. doi: 10.1016/j.ymthe.2005.12.013. Epub 2006 Feb 24. Mol Ther. 2006. PMID: 16500153 Free PMC article.
-
Negative regulation of the alpha interferon-induced antiviral response by the Ras/Raf/MEK pathway.J Virol. 2006 May;80(9):4422-30. doi: 10.1128/JVI.80.9.4422-4430.2006. J Virol. 2006. PMID: 16611902 Free PMC article.
-
"Non-Essential" Proteins of HSV-1 with Essential Roles In Vivo: A Comprehensive Review.Viruses. 2020 Dec 23;13(1):17. doi: 10.3390/v13010017. Viruses. 2020. PMID: 33374862 Free PMC article. Review.
-
The herpes simplex virus type 2 gene ICP10PK protects from apoptosis caused by nerve growth factor deprivation through inhibition of caspase-3 activation and XIAP up-regulation.J Neurochem. 2007 Oct;103(1):365-79. doi: 10.1111/j.1471-4159.2007.04745.x. J Neurochem. 2007. PMID: 17877640 Free PMC article.
-
Manipulation of apoptosis and necroptosis signaling by herpesviruses.Med Microbiol Immunol. 2015 Jun;204(3):439-48. doi: 10.1007/s00430-015-0410-5. Epub 2015 Apr 1. Med Microbiol Immunol. 2015. PMID: 25828583 Free PMC article. Review.
References
-
- Ali, M. A., S. S. Prakash, and R. J. Jariwalla. 1992. Localization of the antigenic sites and intrinsic protein kinase domain within a 300 amino acid segment of the ribonucleotide reductase large subunit from herpes simplex virus type 2. Virology 187:360–367. - PubMed
-
- Alkondon, M., and E. X. Albuquerque. 1993. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J. Pharmacol. Exp. Ther. 265:1455–1473. - PubMed
-
- Ankarcrona, M., J. M. Dypbukt, E. Bonfoco, B. Zhivotovsky, S. Orrenius, S. A. Lipton, and P. Nicoterra. 1995. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15:961–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

