Molecular analysis of mutations in DNA polymerase eta in xeroderma pigmentosum-variant patients
- PMID: 11773631
- PMCID: PMC117388
- DOI: 10.1073/pnas.022473899
Molecular analysis of mutations in DNA polymerase eta in xeroderma pigmentosum-variant patients
Abstract
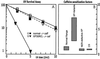
Xeroderma pigmentosum variant (XP-V) cells are deficient in their ability to synthesize intact daughter DNA strands after UV irradiation. This deficiency results from mutations in the gene encoding DNA polymerase eta, which is required for effecting translesion synthesis (TLS) past UV photoproducts. We have developed a simple cellular procedure to identify XP-V cell strains, and have subsequently analyzed the mutations in 21 patients with XP-V. The 16 mutations that we have identified fall into three categories. Many of them result in severe truncations of the protein and are effectively null alleles. However, we have also identified five missense mutations located in the conserved catalytic domain of the protein. Extracts of cells falling into these two categories are defective in the ability to carry out TLS past sites of DNA damage. Three mutations cause truncations at the C terminus such that the catalytic domains are intact, and extracts from these cells are able to carry out TLS. From our previous work, however, we anticipate that protein in these cells will not be localized in the nucleus nor will it be relocalized into replication foci during DNA replication. The spectrum of both missense and truncating mutations is markedly skewed toward the N-terminal half of the protein. Two of the missense mutations are predicted to affect the interaction with DNA, the others are likely to disrupt the three-dimensional structure of the protein. There is a wide variability in clinical features among patients, which is not obviously related to the site or type of mutation.
Figures




References
-
- Kraemer K H, Lee M M, Scotto J. Arch Dermatol. 1987;123:241–250. - PubMed
-
- Bootsma D, Kraemer K H, Cleaver J E, Hoeijmakers J H J. In: The Genetic Basis of Human Cancer. Vogelstein B, Kinzler K W, editors. New York: McGraw–Hill; 1998. pp. 245–274.
-
- Berneburg M, Lehmann A R. Advances in Genetics. Vol. 43. San Diego: Academic; 2001. pp. 71–102. - PubMed
-
- Maher V M, Ouellette L M, Curren R D, McCormick J J. Nature (London) 1976;261:593–595. - PubMed
-
- Myhr B C, Turnbull D, DiPaolo J A. Mutat Res. 1979;62:341–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

