Mullerian-inhibiting substance regulates NF-kappa B signaling in the prostate in vitro and in vivo
- PMID: 11773638
- PMCID: PMC117545
- DOI: 10.1073/pnas.221599298
Mullerian-inhibiting substance regulates NF-kappa B signaling in the prostate in vitro and in vivo
Abstract
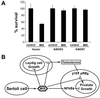
Mullerian-inhibiting substance (MIS) is a member of the transforming growth factor beta superfamily, a class of molecules that regulates growth, differentiation, and apoptosis in many cells. MIS type II receptor in the Mullerian duct is temporally and spatially regulated during development and becomes restricted to the most caudal ends that fuse to form the prostatic utricle. In this article, we have demonstrated MIS type II receptor expression in the normal prostate, human prostate cancer cell lines, and tissue derived from patients with prostate adenocarcinomas. MIS induced NF-kappaB DNA binding activity and selectively up-regulated the immediate early gene IEX-1S in both androgen-dependent and independent human prostate cancer cells in vitro. Dominant negative IkappaBalpha expression ablated both MIS-induced increase of IEX-1S mRNA and inhibition of growth, indicating that activation of NF-kappaB signaling was required for these processes. Androgen also induced NF-kappaB DNA binding activity in prostate cancer cells but without induction of IEX-1S mRNA, suggesting that MIS-mediated increase in IEX-1S was independent of androgen-mediated signaling. Administration of MIS to male mice induced IEX-1S mRNA in the prostate in vivo, suggesting that MIS may function as an endogenous hormonal regulator of NF-kappaB signaling and growth in the prostate gland.
Figures


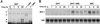
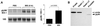
References
-
- Teixeira J, Donahoe P K. J Androl. 1996;17:336–341. - PubMed
-
- Teixeira J, He W W, Shah P C, Morikawa N, Lee M M, Catlin E A, Hudson P L, Wing J, Maclaughlin D T, Donahoe P K. Endocrinology. 1996;137:160–165. - PubMed
-
- Catlin E A, Tonnu V C, Ebb R G, Pacheco B A, Manganaro T F, Ezzell R M, Donahoe P K, Teixeira J. Endocrinology. 1997;138:790–796. - PubMed
-
- Segev D L, Ha T U, Tran T T, Kenneally M, Harkin P, Jung M, MacLaughlin D T, Donahoe P K, Maheswaran S. J Biol Chem. 2000;275:28371–28379. - PubMed
-
- Segev D L, Hoshiya Y, Stephen A E, Hoshiya M, Tran T T, MacLaughlin D T, Donahoe P K, Maheswaran S. J Biol Chem. 2001;276:26799–26806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

