Bromelain reversibly inhibits invasive properties of glioma cells
- PMID: 11774029
- PMCID: PMC1506565
- DOI: 10.1038/sj.neo.7900196
Bromelain reversibly inhibits invasive properties of glioma cells
Abstract
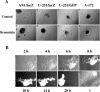
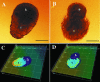
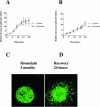
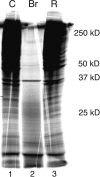
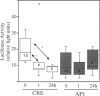
Bromelain is an aqueous extract from pineapple stem that contains proteinases and exhibits pleiotropic therapeutic effects, i.e., antiedematous, antiinflammatory, antimetastatic, antithrombotic, and fibrinolytic activities. In this study, we tested bromelain's effects on glioma cells to assess whether bromelain could be a potential contributor to new antiinvasive strategies for gliomas. Several complementary assays demonstrated that bromelain significantly and reversibly reduced glioma cell adhesion, migration, and invasion without affecting cell viability, even after treatment periods extending over several months. Immunohistochemistry and immunoblotting experiments demonstrated that alpha3 and beta1 integrin subunits and hyaluronan receptor CD44 protein levels were reduced within 24 hours of bromelain treatment. These effects were not reflected at the RNA level because RNA profiling did not show any significant effects on gene expression. Interestingly, metabolic labelling with 35-S methionine demonstrated that de novo protein synthesis was greatly attenuated by bromelain, in a reversible manner. By using a transactivating signaling assay, we found that CRE-mediated signaling processes were suppressed. These results indicate that bromelain exerts its antiinvasive effects by proteolysis, signaling cascades, and translational attenuation.
Figures
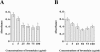


References
-
- Russell DS, Rubinstein LJ. Pathology of Tumours of the Nervous System. 5th ed. London: Williams and Wilkins; 1989. Tumours of Central Neuroepithelial Origin; pp. 83–350.
-
- Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. - PubMed
-
- Rutka JT, Apodaca G, Stern R, Rosenblum M. The extracellular matrix of the central and peripheral nervous systems: structure and function. J Neurosurg. 1988;69:155–170. - PubMed
-
- Uhm JH, Gladson CL, Rao JS. The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:D188–D199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
