Imaging TCR-dependent NFAT-mediated T-cell activation with positron emission tomography in vivo
- PMID: 11774030
- PMCID: PMC1506564
- DOI: 10.1038/sj.neo.7900204
Imaging TCR-dependent NFAT-mediated T-cell activation with positron emission tomography in vivo
Abstract
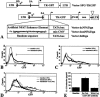
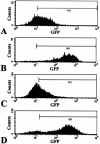
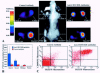
A noninvasive method for molecular imaging of T-cell activity in vivo would be of considerable value. It would aid in understanding the role of specific genes and signal transduction pathways in the course of normal and pathologic immune responses, and could elucidate temporal dynamics and immune regulation at different stages of disease and following therapy. We developed and assessed a novel method for monitoring the T-cell receptor (TCR)-dependent nuclear factor of activated T cells (NFAT)-mediated activation of T cells by optical fluorescence imaging (OFI) and positron emission tomography (PET). The herpes simplex virus type 1 thymidine kinase/green fluorescent protein [HSV1-tk/GFP (TKGFP)] dual reporter gene was used to monitor NFAT-mediated transcriptional activation in human Jurkat cells. A recombinant retrovirus bearing the NFAT-TKGFP reporter system was constructed in which the TKGFP reporter gene was placed under control of an artificial cis-acting NFAT-specific enhancer. Transduced Jurkat cells were used to establish subcutaneous infiltrates in nude rats. We demonstrated that noninvasive OFI and nuclear imaging of T-cell activation is feasible using the NFAT-TKGFP reporter system. PET imaging with [(124)I]FIAU using the NFAT-TKGFP reporter system is sufficiently sensitive to detect T-cell activation in vivo. PET images were confirmed by independent measurements of T-cell activation (e.g., CD69) and induction of GFP fluorescence. PET imaging of TCR-induced NFAT-dependent transcriptional activity may be useful in the assessment of T cell responses, T-cell-based adoptive therapies, vaccination strategies and immunosuppressive drugs.
Figures


References
-
- Tjuvajev JG, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, Safer M, Beattie B, DiResta G, Daghighian F, Augensen F, Koutcher J, Zweit J, Humm J, Larson SM, Finn R, Blasberg R. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. - PubMed
-
- Blasberg RG, Tjuvajev JG. Herpes simplex virus thymidine kinase as a marker/reporter gene for PET imaging of gene therapy. Q J Nucl Med. 1999;43:163–169. - PubMed
-
- Jacobs A, Tjuvajev JG, Dubrovin M, Akhurst T, Balatoni J, Beattie B, Joshi R, Finn R, Larson SM, Herrlinger U, Pechan PA, Chiocca EA, Breakefield XO, Blasberg RG. Positron emission tomography-based imaging of transgene expression mediated by replication-conditional, oncolytic herpes simplex virus type 1 mutant vectors in vivo. Cancer Res. 2001;61:2983–2995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
