TRAIL/Apo-2L: mechanisms and clinical applications in cancer
- PMID: 11774036
- PMCID: PMC1506567
- DOI: 10.1038/sj.neo.7900203
TRAIL/Apo-2L: mechanisms and clinical applications in cancer
Abstract
TNF-related apoptosis-inducing ligand (TRAIL/APO-2L) is a member of the TNF family that promotes apoptosis by binding to the transmembrane receptors TRAIL-R1/DR4 and TRAIL-R2/DR5. Its cytotoxic activity is relatively selective to the human tumor cell lines without much effect on the normal cells. Hence, it exerts an antitumor activity without causing toxicity, as apparent by studies with several xenograft models. This review discusses the intracellular mechanisms by which TRAIL induces apoptosis. The major pathway of its action proceeds through the formation of DISC and activation of caspase-8. The apoptotic processes, therefore, follow two signaling pathways, namely the mitochondrial-independent activation of caspase-3, and mitochondrial-dependent apoptosis due to cleavage of BID by caspase-8, the formation of apoptosomes, and activation of caspase-9 and the downstream caspases. Bcl-2 and Bcl-X(L) have no effect on TRAIL-induced apoptosis in lymphoid cells, whereas these genes block or delay apoptosis in nonlymphoid cancer cells. TRAIL participates in cytotoxicity mediated by activated NK cells, monocytes, and some cytotoxic T cells. Hence, TRAIL may prove to be an effective antitumor agent. In addition, it may enhance the effectiveness of treatment with chemotherapeutic drugs and irradiation. Nontagged Apo-2L/TRAIL does not cause hepatotoxicity in monkeys and chimpanzees and in normal human hepatocytes. Thus, nontagged Apo-2L/TRAIL appears to be a promising new candidate for use in the treatment of cancer.
Figures
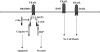

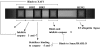

References
-
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. - PubMed
-
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. - PubMed
-
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. - PMC - PubMed
-
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. - PubMed
-
- Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
