Host cell responses to genotypically similar Helicobacter pylori isolates from United States and Japan
- PMID: 11777849
- PMCID: PMC119904
- DOI: 10.1128/cdli.9.1.167-175.2002
Host cell responses to genotypically similar Helicobacter pylori isolates from United States and Japan
Abstract
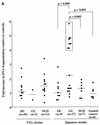
Associations of Helicobacter pylori genotypes with disease differ between Western countries and Asia. Therefore, we directly compared histopathological and in vitro responses to clinical isolates with similar genotypes. Sixty-three cagA(+) vacAs1/m1 H. pylori isolates (United States, n = 24; Japan, n = 39) and eight cagA-negative vacAs2/m2 strains were incubated with AGS cells, and supernatants were assayed for interleukin-8 (IL-8) and for DNA fragmentation. CagA tyrosine phosphorylation in AGS cells and the sequence of the putative HP0638 (oipA) signal sequence region were determined for 22 representative strains. HP0638 and/or cag island mutant strains were created and examined in IL-8 and CagA tyrosine phosphorylation assays. Levels of IL-8 induction and DNA fragmentation were similar in the U.S. and Japanese cagA(+) vacAs1/m1 isolates. All 10 of the isolates with the highest IL-8 induction and 8 of the 10 isolates with the lowest IL-8 induction had an in-frame oipA open reading frame, and all 10 of the isolates with the highest IL-8 induction and 7 of the 10 isolates with the lowest IL-8 induction induced CagA tyrosine phosphorylation in AGS cells. Eight isolates from gastric ulcer patients induced significantly more apoptosis in vitro, and more severe gastritis and atrophy in vivo, than other Japanese isolates. Disruption of HP0638 did not affect IL-8 induction or CagA tyrosine phosphorylation. Thus, H. pylori cagA(+) vacAs1/m1 isolates from the United States and Japan induce similar IL-8 and apoptosis levels. Inactivation of HP0638 does not alter epithelial responses mediated by the cag island in vitro. Assessment of apoptosis in vitro identified a group of H. pylori isolates that induce more severe gastric inflammation and atrophy.
Figures
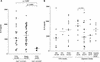


References
-
- Achtman, M., T. Azuma, D. E. Berg, Y. Ito, G. Morelli, Z. J. Pan, S. Suerbaum, S. A. Thompson, A. van der Ende, and L. J. van Doorn. 1999. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol. Microbiol. 32:459–470. - PubMed
-
- Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37–53. - PubMed
-
- Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176–180. - PubMed
-
- Ando, T., K. Kusugami, M. Ohsuga, M. Shinoda, M. Sakakibara, H. Saito, A. Fukatsu, S. Ichiyama, and M. Ohta. 1996. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am. J. Gastroenterol. 91:1150–1156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

