Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue
- PMID: 11781332
- PMCID: PMC2173594
- DOI: 10.1083/jcb.200111004
Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue
Abstract
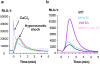
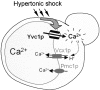
Calcium ions, present inside all eukaryotic cells, are important second messengers in the transduction of biological signals. In mammalian cells, the release of Ca(2+) from intracellular compartments is required for signaling and involves the regulated opening of ryanodine and inositol-1,4,5-trisphosphate (IP3) receptors. However, in budding yeast, no signaling pathway has been shown to involve Ca(2+) release from internal stores, and no homologues of ryanodine or IP3 receptors exist in the genome. Here we show that hyperosmotic shock provokes a transient increase in cytosolic Ca(2+) in vivo. Vacuolar Ca(2+), which is the major intracellular Ca(2+) store in yeast, is required for this response, whereas extracellular Ca(2+) is not. We aimed to identify the channel responsible for this regulated vacuolar Ca(2+) release. Here we report that Yvc1p, a vacuolar membrane protein with homology to transient receptor potential (TRP) channels, mediates the hyperosmolarity induced Ca(2+) release. After this release, low cytosolic Ca(2+) is restored and vacuolar Ca(2+) is replenished through the activity of Vcx1p, a Ca(2+)/H(+) exchanger. These studies reveal a novel mechanism of internal Ca(2+) release and establish a new function for TRP channels.
Figures


References
-
- Albertyn, J., S. Hohmann, J.M. Thevelein, and B.A. Prior. 1994. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 14:4135–4144. - PMC - PubMed
-
- Batiza, A.F., T. Schulz, and P.H. Masson. 1996. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 271:23357–23362. - PubMed
-
- Beeler, T., K. Gable, and T. Dunn. 1997. Activation of divalent cation influx into S. cerevisiae cells by hypotonic downshift. J. Membr. Biol. 160:77–83. - PubMed
-
- Belde, P.J.M., J.H. Vossen, G.W.F.H. Borst-Pauwels, and A.P.R. Theuvenet. 1993. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of Saccharomyces cerevisiae. FEBS Lett. 323:113–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

