Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis
- PMID: 11781347
- PMCID: PMC150815
- DOI: 10.1172/JCI12035
Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis
Abstract
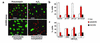
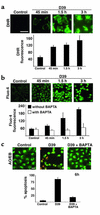
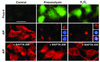
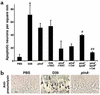
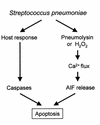
Pneumococcus is the most common and aggressive cause of bacterial meningitis and induces a novel apoptosis-inducing factor-dependent (AIF-dependent) form of brain cell apoptosis. Loss of production of two pneumococcal toxins, pneumolysin and H(2)O(2), eliminated mitochondrial damage and apoptosis. Purified pneumolysin or H(2)O(2) induced microglial and neuronal apoptosis in vitro. Both toxins induced increases of intracellular Ca(2+) and triggered the release of AIF from mitochondria. Chelating Ca(2+) effectively blocked AIF release and cell death. In experimental pneumococcal meningitis, pneumolysin colocalized with apoptotic neurons of the hippocampus, and infection with pneumococci unable to produce pneumolysin and H(2)O(2) significantly reduced damage. Two bacterial toxins, pneumolysin and, to a lesser extent, H(2)O(2), induce apoptosis by translocation of AIF, suggesting new neuroprotective strategies for pneumococcal meningitis.
Figures


References
-
- Bohr V, Paulson OB, Rasmussen N. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch Neurol. 1984; 41:1045–1049. - PubMed
-
- Durand ML, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993; 328:21–28. - PubMed
-
- Pfister HW, Feiden W, Einhäupl KM. Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch Neurol. 1993; 50:575–581. - PubMed
-
- Zysk G, et al. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol. 1996; 55:722–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

