Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology
- PMID: 11781348
- PMCID: PMC150821
- DOI: 10.1172/JCI13696
Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology
Abstract
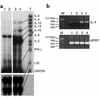
IL-9 is a pleiotropic cytokine with multiple functions on many cell types involved in the pathology of human asthma. The constitutive overexpression of IL-9 in the lungs of transgenic mice resulted in an asthma-like phenotype. To define the contribution of IL-9 to lung inflammation we generated transgenic mice in which lung-specific expression of the IL-9 transgene is inducible by doxycycline. Transgene induction resulted in lymphocytic and eosinophilic infiltration of the lung, airway epithelial cell hypertrophy with mucus production, and mast cell hyperplasia, similar to that seen in mice that constitutively expressed IL-9 in their lungs. Various cytokines, including IL-4, IL-5, and IL-13, were expressed in the lung in response to IL-9. Blockade of IL-4 or IL-5 following IL-9 induction reduced airway eosinophilia without affecting mucus production. In contrast, neutralization of IL-13 completely abolished both lung inflammation and mucus production. These findings suggest that pathologic changes in the lung require additional signals beyond IL-9, provided by IL-4, IL-5, and IL-13, to develop fully.
Figures


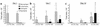






References
-
- Holgate ST. The inflammatory basis of asthma and its implications for drug treatment. Clin Exp Allergy. 1996; 4(Suppl.):1–4. - PubMed
-
- Lukacs NW, Strieter RM, Kunkel SL. Leukocyte infiltration in allergic airway inflammation. Am J Respir Cell Mol Biol. 1995; 13:1–6. - PubMed
-
- Robinson DS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992; 326:298–304. - PubMed
-
- Riffo-Vasquez Y, Pitchford S, Spina D. Cytokines in airway inflammation. Int J Biochem Cell Biol. 2000; 32:833–853. - PubMed
-
- Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993; 189:419–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

