Targeted expression of a human pituitary tumor-derived isoform of FGF receptor-4 recapitulates pituitary tumorigenesis
- PMID: 11781352
- PMCID: PMC150823
- DOI: 10.1172/JCI14036
Targeted expression of a human pituitary tumor-derived isoform of FGF receptor-4 recapitulates pituitary tumorigenesis
Retraction in
-
Retraction. Targeted expression of a human pituitary tumor-derived isoform of FGF receptor-4 recapitulates pituitary tumorigenesis.J Clin Invest. 2015 Aug 3;125(8):3303. doi: 10.1172/JCI83399. Epub 2015 Aug 3. J Clin Invest. 2015. PMID: 26237043 Free PMC article. No abstract available.
Abstract
It is estimated that up to one in five individuals develop pituitary gland tumors. Despite the common occurrence of these tumors, the pathogenetic mechanisms underlying their development remain largely unknown. We report the identification of a novel pituitary tumor-derived, N-terminally truncated isoform of FGF receptor-4 (ptd-FGFR4). The corresponding mRNA results from alternative transcription initiation and encodes a polypeptide that lacks a signal peptide and the first two extracellular Ig-like domains. ptd-FGFR4 has a distinctive cytoplasmic residence, is constitutively phosphorylated, and is transforming in vitro and in vivo. Here we show that targeted expression of ptd-FGFR4, but not FGFR4, results in pituitary tumors that morphologically recapitulate the human disease.
Figures
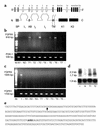
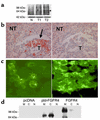

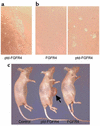
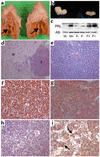
Comment in
-
Pituitary adenomas in man and mouse: oncogenic potential of a truncated fibroblast growth factor receptor 4.J Clin Invest. 2002 Jan;109(1):15-6. doi: 10.1172/JCI14772. J Clin Invest. 2002. PMID: 11781345 Free PMC article. No abstract available.
Similar articles
-
Expression of pituitary tumour-derived, N-terminally truncated isoform of fibroblast growth factor receptor 4 (ptd-FGFR4) correlates with tumour invasiveness but not with G-protein alpha subunit (gsp) mutation in human GH-secreting pituitary adenomas.Clin Endocrinol (Oxf). 2008 Mar;68(3):435-41. doi: 10.1111/j.1365-2265.2007.03062.x. Epub 2007 Dec 7. Clin Endocrinol (Oxf). 2008. PMID: 18070145
-
Pituitary tumor-derived fibroblast growth factor receptor 4 isoform disrupts neural cell-adhesion molecule/N-cadherin signaling to diminish cell adhesiveness: a mechanism underlying pituitary neoplasia.Mol Endocrinol. 2004 Oct;18(10):2543-52. doi: 10.1210/me.2004-0182. Epub 2004 Jul 1. Mol Endocrinol. 2004. PMID: 15231874
-
Targeting N-cadherin through fibroblast growth factor receptor-4: distinct pathogenetic and therapeutic implications.Mol Endocrinol. 2006 Nov;20(11):2965-75. doi: 10.1210/me.2006-0223. Epub 2006 Jul 20. Mol Endocrinol. 2006. PMID: 16857743
-
Chromatin remodeling and histone modifications in pituitary tumors.Mol Cell Endocrinol. 2010 Sep 15;326(1-2):66-70. doi: 10.1016/j.mce.2009.12.028. Epub 2010 Jan 7. Mol Cell Endocrinol. 2010. PMID: 20060434 Review.
-
Genetics and proteomics of pituitary tumors.Endocrine. 2005 Oct;28(1):43-7. doi: 10.1385/ENDO:28:1:043. Endocrine. 2005. PMID: 16311409 Review.
Cited by
-
Pituitary adenomas: historical perspective, surgical management and future directions.CNS Oncol. 2015;4(6):411-29. doi: 10.2217/cns.15.21. Epub 2015 Oct 26. CNS Oncol. 2015. PMID: 26497533 Free PMC article. Review.
-
FGFR4 role in epithelial-mesenchymal transition and its therapeutic value in colorectal cancer.PLoS One. 2013 May 16;8(5):e63695. doi: 10.1371/journal.pone.0063695. Print 2013. PLoS One. 2013. PMID: 23696849 Free PMC article.
-
Epidemiology and etiopathogenesis of pituitary adenomas.J Neurooncol. 2014 May;117(3):379-94. doi: 10.1007/s11060-013-1354-5. Epub 2014 Jan 31. J Neurooncol. 2014. PMID: 24481996 Review.
-
The role of MMP-1 and FGFR4-R388 gene polymorphisms in pituitary adenoma.Acta Med Litu. 2017;24(4):177-190. doi: 10.6001/actamedica.v24i4.3613. Acta Med Litu. 2017. PMID: 29487481 Free PMC article.
-
Pituitary somatolactotropes evade an oncogenic response to Ras.Mol Cell Endocrinol. 2018 Nov 15;476:165-172. doi: 10.1016/j.mce.2018.05.006. Epub 2018 May 9. Mol Cell Endocrinol. 2018. PMID: 29753028 Free PMC article.
References
-
- Asa, S.L. 1998. Tumors of the pituitary gland. Atlas of tumor pathology, series 3, fascicle 22. Armed Forces Institute of Pathology. Washington, DC, USA. 214 pp.
-
- Asa SL, Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr Rev. 1998; 19:798–827. - PubMed
-
- Jacks T, et al. Effects of an Rbmutation in the mouse. Nature. 1992; 359:295–300. - PubMed
-
- Kelly MA, et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997; 19:103–113. - PubMed
-
- Asa SL, Kelly MA, Grandy DK, Low MJ. Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology. 1999; 140:5348–5355. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

