Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions
- PMID: 11781364
- PMCID: PMC2196015
- DOI: 10.1084/jem.20011145
Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions
Abstract
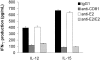
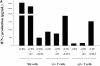
Infection with hepatitis C virus (HCV) is a leading cause of chronic liver disease worldwide. Little is known about how this virus is able to persist or whether this persistence might be because of its ability to alter the early innate immune response. The major HCV envelope protein E2 has been shown to bind to CD81. Thus, HCV binding to natural killer (NK) cells could result in the cross-linking of CD81. To explore this possibility, we investigated whether cross-linking CD81 on NK cells could alter NK cell function. CD81 cross-linking by monoclonal antibody (mAb) specific for CD81 or by immobilized E2 have been shown to result in costimulatory signals for human T cells. In this study, we show that CD81 cross-linking via immobilized E2 or mAbs specific for CD81 inhibits not only non major histocompatibility complex-restricted cytotoxicity mediated by NK cells but also interferon (IFN)-gamma production by NK cells after exposure to interleukin (IL)-2, IL-12, IL-15, or CD16 cross-linking. These results show that CD81 cross-linking mediates completely different signals in NK cells versus T cells. Importantly, these results suggest that one mechanism whereby HCV can alter host defenses and innate immunity is via the early inhibition of IFN-gamma production by NK cells.
Figures
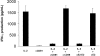



Similar articles
-
Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein.J Exp Med. 2002 Jan 7;195(1):35-41. doi: 10.1084/jem.20011124. J Exp Med. 2002. PMID: 11781363 Free PMC article.
-
Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection.J Hepatol. 2010 Feb;52(2):183-90. doi: 10.1016/j.jhep.2009.11.003. Epub 2009 Dec 16. J Hepatol. 2010. PMID: 20015567
-
Activation of natural killer cells by hepatitis C virus particles in vitro.Clin Exp Immunol. 2011 Sep;165(3):352-62. doi: 10.1111/j.1365-2249.2011.04431.x. Epub 2011 Jun 17. Clin Exp Immunol. 2011. PMID: 21682720 Free PMC article.
-
Lymphocyte distribution and intrahepatic compartmentalization during HCV infection: a main role for MHC-unrestricted T cells.Arch Immunol Ther Exp (Warsz). 2002;50(5):307-16. Arch Immunol Ther Exp (Warsz). 2002. PMID: 12455864 Review.
-
Hepatitis C virus (HCV): a review of immunological aspects.Int Rev Immunol. 2008;27(6):497-517. doi: 10.1080/08830180802432178. Int Rev Immunol. 2008. PMID: 19065353 Review.
Cited by
-
Inhibition of human natural killer cell activity by influenza virions and hemagglutinin.J Virol. 2010 May;84(9):4148-57. doi: 10.1128/JVI.02340-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164232 Free PMC article.
-
Strategies for targeting tetraspanin proteins: potential therapeutic applications in microbial infections.BioDrugs. 2009;23(6):341-59. doi: 10.2165/11315650-000000000-00000. BioDrugs. 2009. PMID: 19894777 Free PMC article. Review.
-
The Influence of Hepatitis C Viral Loads on Natural Killer Cell Function.Gastroenterology Res. 2019 Feb;12(1):8-15. doi: 10.14740/gr1081w. Epub 2019 Feb 26. Gastroenterology Res. 2019. PMID: 30834029 Free PMC article.
-
Natural killer cell function and dysfunction in hepatitis C virus infection.Biomed Res Int. 2014;2014:903764. doi: 10.1155/2014/903764. Epub 2014 Jun 25. Biomed Res Int. 2014. PMID: 25057504 Free PMC article. Review.
-
Hepatitis C Virus E2 Protein Induces Upregulation of IL-8 Pathways and Production of Heat Shock Proteins in Human Thyroid Cells.J Clin Endocrinol Metab. 2017 Feb 1;102(2):689-697. doi: 10.1210/jc.2016-3403. J Clin Endocrinol Metab. 2017. PMID: 27860532 Free PMC article.
References
-
- Houghton, M. 1996. Hepatitis C viruses. Virology. B.N. Fields, D.M. Knipe, and P.M. Howley, editors. Lippincott-Raven, Philadelphia. 1035–1058 pp.
-
- Cohen, J. 1999. The scientific challenge of hepatitis C virus. Science. 285:26–30. - PubMed
-
- World Health Organization. 1997. Hepatitis C viruses. Wkly. Epidemiol. Rec. 72:65–72. - PubMed
-
- Weiner, A.J., H.M. Geysen, C. Christopherson, J.E. Hall, T.J. Mason, G. Sarraco, F. Bonino, K. Crowford, C.D. Marion, K.A. Crowford, et al. 1992. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc. Natl. Acad. Sci. USA. 89:3468–3473. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

