Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I
- PMID: 11784785
- PMCID: PMC6758668
- DOI: 10.1523/JNEUROSCI.22-02-00404.2002
Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I
Abstract
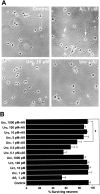
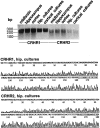
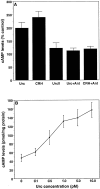
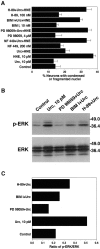
Urocortin and urocortin II are members of the corticotropin-releasing hormone (CRH) family of neuropeptides that function to regulate stress responses. Two high-affinity G-protein-coupled receptors have been identified that bind CRH and/or urocortin I and II, designated CRHR1 and CRHR2, both of which are present in hippocampal regions of mammalian brain. The hippocampus plays an important role in regulating stress responses and is a brain region in which neurons are vulnerable during disease and stress conditions, including cerebral ischemia, Alzheimer's disease, and anxiety disorders. Here we report that urocortin exerts a potent protective action in cultured rat hippocampal neurons with concentrations in the range of 0.5-5.0 pm, increasing the resistance of the cells to oxidative (amyloid beta-peptide, 4-hydroxynonenal, ferrous sulfate) and excitotoxic (glutamate) insults. We observed that urocortin is 10-fold more potent than CRH in protecting hippocampal neurons from insult, whereas urocortin II is ineffective. RT-PCR and sequencing analyses revealed the presence of both CRHR1 and CRHR2 in the hippocampal cultures, with CRHR1 being expressed at much higher levels than CRHR2. Using subtype-selective CRH receptor antagonists, we provide evidence that the neuroprotective effect of exogenously added urocortin is mediated by CRHR1. Furthermore, we provide evidence that the signaling pathway that mediates the neuroprotective effect of urocortin involves cAMP-dependent protein kinase, protein kinase C, and mitogen-activated protein kinase. This is the first demonstration of a biological activity of urocortin in hippocampal neurons, suggesting a role for the peptide in adaptive responses of hippocampal neurons to potentially lethal oxidative and excitotoxic insults.
Figures



Similar articles
-
Corticotropin-releasing hormone protects neurons against insults relevant to the pathogenesis of Alzheimer's disease.Neurobiol Dis. 2001 Jun;8(3):492-503. doi: 10.1006/nbdi.2001.0395. Neurobiol Dis. 2001. PMID: 11442356
-
Brain region-specific neuroprotective action and signaling of corticotropin-releasing hormone in primary neurons.Endocrinology. 2003 Sep;144(9):4051-60. doi: 10.1210/en.2003-0168. Endocrinology. 2003. PMID: 12933679
-
Corticotropin-releasing factor (CRF) and related peptides confer neuroprotection via type 1 CRF receptors.Neuropharmacology. 2003 Oct;45(5):623-36. doi: 10.1016/s0028-3908(03)00211-9. Neuropharmacology. 2003. PMID: 12941376
-
Milestones in CRH Research.Curr Mol Pharmacol. 2017;10(4):259-263. doi: 10.2174/1874467210666170109165219. Curr Mol Pharmacol. 2017. PMID: 28071586 Review.
-
Corticotropin-Releasing Hormone Family and Their Receptors in the Cardiovascular System.Circ J. 2019 Jan 25;83(2):261-266. doi: 10.1253/circj.CJ-18-0428. Epub 2018 Dec 22. Circ J. 2019. PMID: 30584229 Review.
Cited by
-
Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory.J Neurosci. 2003 Dec 10;23(36):11436-43. doi: 10.1523/JNEUROSCI.23-36-11436.2003. J Neurosci. 2003. PMID: 14673008 Free PMC article.
-
Mitogen- and Stress-Activated Protein Kinase 1 Regulates Status Epilepticus-Evoked Cell Death in the Hippocampus.ASN Neuro. 2017 Sep-Oct;9(5):1759091417726607. doi: 10.1177/1759091417726607. ASN Neuro. 2017. PMID: 28870089 Free PMC article.
-
Stress and the developing hippocampus: a double-edged sword?Mol Neurobiol. 2003 Apr;27(2):121-36. doi: 10.1385/MN:27:2:121. Mol Neurobiol. 2003. PMID: 12777683 Free PMC article. Review.
-
Class II G protein-coupled receptors and their ligands in neuronal function and protection.Neuromolecular Med. 2005;7(1-2):3-36. doi: 10.1385/nmm:7:1-2:003. Neuromolecular Med. 2005. PMID: 16052036 Free PMC article. Review.
-
Corticotropin-releasing factor and urocortin I activate CREB through functionally selective Gβγ signaling in hippocampal pyramidal neurons.Eur J Neurosci. 2011 Sep;34(5):671-81. doi: 10.1111/j.1460-9568.2011.07812.x. Epub 2011 Aug 8. Eur J Neurosci. 2011. PMID: 21819464 Free PMC article.
References
-
- Bishop GA, Seelandt CM, King JS. Cellular localization of corticotropin-releasing factor receptors in the adult mouse cerebellum. Neuroscience. 2000;101:1083–1092. - PubMed
-
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. - PubMed
-
- Brar BK, Stephanou A, Okosi A, Lawrence KM, Knight RA, Marber MS, Latchman DS. CRH-like peptides protect cardiac myocytes from lethal ischaemic injury. Mol Cell Endocrinol. 1999;158:55–63. - PubMed
-
- Brar BK, Jonassen AK, Stephanou A, Santilli G, Railson J, Knight RA, Yellon DM, Latchman DS. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem. 2000;275:8508–8514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
