Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation
- PMID: 11784802
- PMCID: PMC6758651
- DOI: 10.1523/JNEUROSCI.22-02-00554.2002
Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation
Abstract
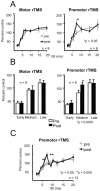
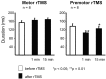
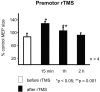
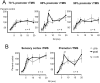
Connections between the premotor cortex and the primary motor cortex are dense and are important in the visual guidance of arm movements. We have shown previously that it is possible to engage these connections in humans and to measure the net amount of inhibition/facilitation from premotor to motor cortex using single-pulse transcranial magnetic stimulation (TMS). The aim of this study was to test whether premotor activation can affect the excitability of circuits within the primary motor cortex (M1) itself. Repetitive TMS (rTMS), which is known to produce effects that outlast the train at the site of stimulation, was given for 20 min at 1 Hz over premotor, primary motor, and sensory areas of cortex at an intensity of 80% of the active motor threshold for the motor hand area. The excitability of some corticocortical connections in M1 was probed by using paired-pulse testing of intracortical inhibition (ICI) and intracortical facilitation (ICF) with a coil placed over the motor cortex hand area. rTMS over the premotor cortex, but not other areas, changed the time course of the ICI/ICF for up to 1 hr afterward without affecting motor thresholds or motor-evoked potential recruitment. The cortical silent period was also shortened. The implication is that rTMS at a site distant from the motor cortex can change the excitability of circuits intrinsic to the motor cortex.
Figures


References
-
- Ashby P, Reynolds C, Wennberg A, Lozano AM, Rothwell JC. On the focal nature of inhibition and facilitation in the human motor cortex. Clin Neurophysiol. 1999;110:550–555. - PubMed
-
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9:132–136. - PubMed
-
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. - PubMed
-
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. NeuroImage. 2001;14:1444–1453. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
